I often take the opportunity to examine the fresh botanical to compare the commercial essential oils. Pink grapefruit proved no exception. During my last trip to Florida I picked up some pink grapefruit from Indian River, a well-known and respected area for delicious pink grapefruit.
Grapefruit has a very distinctive fragrance and flavor. Pink grapefruit has the additional allure of being light pink to Ruby red, which stimulates another of our keenest senses, sight. It is well known that color can influence our perception of flavor and fragrance and that is why professional panelists are often prevented from experiencing any color at all during sensory panel evaluations.
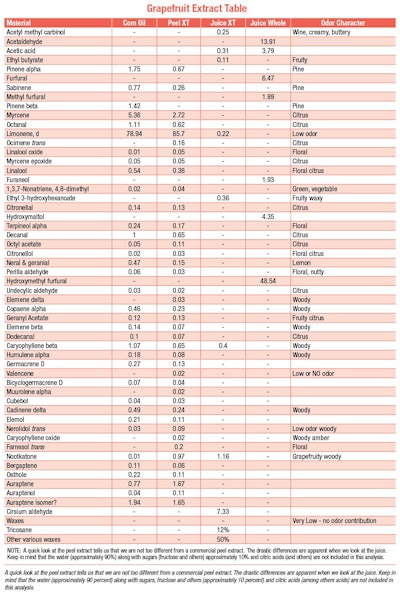
I am perpetually interested in the qualitative as well as quantitative differences between the essential oils that are normally used in our industry – as opposed to fresh fruit peel and commercial juice extracts, which often fail to capture the fresh character of the botanical.
I promptly extracted the peel and juice with methylene chloride and prepped the samples for GCMS analysis. In addition, I examined the juice directly (without extraction) as I am aware that much of the important flavor and fragrance components are in the water (not the peel) section, which exhibit significant olfactory and flavor differences. This had a few analytical surprises that we shall see a bit later.
Additionally, it should be noted that while grapefruit and grapefruit juice are known to be bitter, the bitter flavor does not come from the fruit itself, but the surrounding albedo and section casing very likely from naringen and other flavenoids present in large quantities. If one carefully removes the peel, as well as the skin encasing the sections, one might be pleasantly surprised at how sweet the fruit is and completely devoid of the characteristic bitter flavor that one experiences with grapefruit juice. This might be one of the reasons that grapefruit juice has always taken a back street to orange juice which is much more preferred and consumed in much larger quantities. This of course, affects supply and pricing – basic economics.
Component differences in juice
The first thing that appears as revelatory is the stark contrast between the directly-injected juice and the juice extract. A bit of reflection and understanding of what happens in an injection port (250 °C) and a bit of literature research helps explain this huge difference1.
These results do not indicate the true nature of the juice as it is but points to the reason processed juices are drastically different in character.
When fructose is heated in an acidic medium, the fructose is transformed into hydroxymethyl fufural (HMF) (See F-1)2.
The other major component, cirsium aldehyde is a condensation product of hydroxymethyl furfural (see F-2).
Major contributors in grapefruit juice
Many of the other components, both furfural and furaneol, as well as other pyrones are formed during this process. What is likely to be present in the juice, (pre-reaction state) are acetaldehyde, acetic and other acids which help promote the reactions. Of course, one might expect limonene to be a minor component in the juice and we can see that this is the case, as limonene is very hydrophobic. I should note that limonene is an insignificant contributor to aroma or character and as such might be considered a carrier or flavor delivery substance.
While many oxygenated components and aldehydes are major contributors to the grapefruit aroma, it is the esters that provide the fruity body. However, the most characterizing components in grapefruit are the sulfur-containing constituents, along with nootkatone, that play the most important role for character. It is a combination of several sulfur components that exist in grapefruit, the single most important one is grapefruit mercaptan (see F-3 and F-4).
Without these sulfur containing components, grapefruit would smell very much like orange, which is often used to blend with grapefruit during shortage conditions, which unfortunately happens much too often.
Conclusion
When I have the opportunity to work with natural products and go through a direct extraction and analysis, there are often surprises and lessons to be learned. In this case, the lesson was clear. Fructose, as well as other sugars are often in an acidic medium in the natural product or botanical. The subsequent reaction products of furfural, hydroxy methyl furfural, as well as pyrones, lactones and other related molecules are bound to be formed either naturally, or as a result of thermal or other reaction drivers.
Upon reflection over my own personal history of extraction and analysis, it is evident why so many fruits, which naturally contain fructose, would develop—as part of their aroma and flavor profile—a sweet cooked characteristic. This is usually due to maltol, which we can see is a result of decomposition of sugars in an acidic environment.
In a short sentence, what comes out of a gas chromatograph may not be an indicator of what was put into the injection port.
References:
- Assry, Rajeev S., Mechanistic Insights into the Decomposition of Fructose to HMF. The Journal of Physical Chemistry, ACS Publication 2011
- Rosatella, A. A. (2011). 5-Hydroxymethylfurfural (HMF) as a building block platform: Green Chemistry, 13(4), 754-793.