
The Research Institute for Fragrance Materials, Inc. (RIFM) and scientific modeling and data analytics company Creme Global published a studya comparing the fragrance exposure of the highest-end product users to the internationally recognized Threshold of Toxicological Concern (TTC) and Dermal Sensitization Threshold (DST) to determine a realistic understanding of consumer exposure to fragrance.
Read this in the May issue!
Perfumer & Flavorist+ (P&F+) connected with the study's lead author, senior scientist Isabelle Lee, Ph.D., and co-author RIFM president Anne Marie Api, Ph.D., to discuss the finer points of the study's results and what it means for fragrance industry professionals as well as consumers.
P&F+: Congratulations on publishing the Fragrance Ingredient Exposure Paper! Can you share some key points/takeaways of the results in layperson’s terms?
RIFM Senior Scientist Isabelle Lee, Ph.D.: Thank you so much! Our primary finding won’t surprise many fragrance formulators or related professionals: Even the highest-end users of fragranced products have extremely low exposure to fragrance. In fact, high-end consumer exposure to most fragrances, even considering all products used, is orders of magnitude below levels the international scientific community recognizes as safe.
P&F+: Can you talk more about those internationally recognized safe-use levels?
Isabelle Lee [IL]: Absolutely; there are two of them: The TTC, short for Threshold of Toxicological Concern, and the DST, or Dermal Sensitization Threshold.
The TTC refers to safe-use exposure levels below which there would be no appreciable risk to human health. There is a TTC for the respiratory tract (called “inhalation” exposure) or other sites within the body, for example, the reproductive system, or “systemic” effects from repeated exposure.
The DST refers to safe-use levels for any exposure via the skin.
The TTC (and, by extension, the DST) originated with the U.S. Food and Drug Administration’s Threshold of Regulation (ToR). The TTC expands upon the FDA’s ToR and considers all study data and the actual molecular structure of the ingredient to arrive at a level beneath which exposure would present no cause for concern.
The TTC approach is recognized by regulators around the world, including the European Food Safety Authority (EFSA).
P&F+: So, RIFM keeps track of consumer exposure to fragrances?
Anne Marie Api [AMA]: Yes. In fact, we could not conduct risk assessments without knowing the highest-end consumers’ exposure to all in-use fragrance ingredients.
That’s why RIFM and data analytics software leader Creme Global developed the Creme-RIFM Aggregate Exposure Model. The model uses real-world industry use levels and habits & practices data from over 40,000 consumers in Europe, North America and Asia.
P&F+: Does “aggregate exposure” cover everything?
AMA: Yes. It refers to the total combined amount of a single fragrance ingredient that a person comes in contact with through all consumer products used daily.
The model contains data on all fragrance materials in the RIFM Safety Assessment Program and includes personal care, cosmetics, household and air care products. A total of 72 products are in the model.
P&F+: What impact does the model have on perfumers?
IL: We couldn’t perform risk-based safety assessments without it. Without risk analysis, one only has hazard as a metric, which in some scenarios could mean a ban on everything in the palette.
When a risk assessment concludes that risk management is required, the assessment will include maximum acceptable concentration (MAC) values broken down by fragranced consumer product type. These MAC values serve as the basis of the International Fragrance Association (IFRA) Standards.
While some may experience these standards as limiting, without them, the alternative might be a complete ban on ingredients that would be otherwise available for responsible safe use.
P&F+: Doesn’t acknowledging hazards ensure safety?
AMA: It’s a common misunderstanding. Water and oxygen, substances we could not live without, are both hazards because they can cause us harm. Drinking too much water can induce water poisoning, and inhaling too much oxygen can result in oxygen toxicity; both are potentially fatal.
Knowing that something is a hazard is insufficient to ensure safe consumption. Scientists need to understand its risks.
P&F+: And that’s where the low-exposure paper comes in.
IL: Exactly. RIFM has published peer-reviewed risk assessments covering nearly 2,000 of the discrete lab-produced fragrance ingredients, and we expect to have covered the entire palette of 3,000 ingredients, including naturals, by 2027.
In doing all this work, we realized that exposure to fragrance ingredients was low, but we hadn’t looked at the entire palette at once, which we did for this paper.
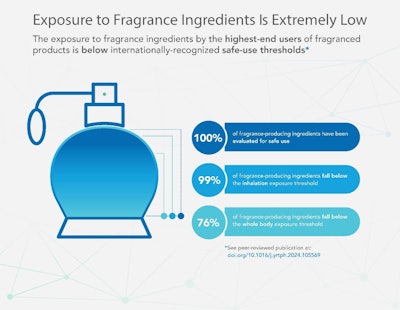
 The data showed that, for 76% of fragrance ingredients, the systemic exposure experienced by the highest-end users falls below the TTC, while the inhalation exposure and 99% of fragrance ingredients fall below TTC levels.Adobe Stock
The data showed that, for 76% of fragrance ingredients, the systemic exposure experienced by the highest-end users falls below the TTC, while the inhalation exposure and 99% of fragrance ingredients fall below TTC levels.Adobe Stock
P&F+: Were there any unexpected nuances that you discovered?
IL: The data showed that, for 76% of fragrance ingredients, the systemic exposure experienced by the highest-end users falls below the TTC, while the inhalation exposure and 99% of fragrance ingredients fall below TTC levels.
We also determined that household and air care products have the lowest exposure by product type. In contrast, personal care/cosmetic products have the highest exposures by product type.
What do these results mean for perfumers?
AMA: A byproduct of all RIFM’s work is that perfumers can confidently create new and exciting scent blends without worrying about causing harm via consumer or environmental exposure.
Just as science has formed the bedrock of perfumery since the dawn of the art, science further supports the perfumer by providing bonafide risk analyses of ingredients. Without peer-reviewed and publicly available risk analyses to support them, many ingredients face removal from the palette by governmental agencies lacking the resources to perform the difficult, expensive science RIFM has undertaken for the last half-century.
P&F+: Can you share any plans for the next phase of this study?
IL: The Creme-RIFM Model is consistently updated to refine our understanding of exposure. We survey each fragrance ingredient at least every five years. We survey the consumer product use and “habits and practices of use” data every six years. For the fragrance exposure data to be realistic, it must remain current. In addition, RIFM and Creme are investigating how to expand the model to other regions of the world.
P&F+: What can World Perfumery Congress attendees experience at the RIFM booth this year?
AMA: RIFM’s chief operating officer, Steven Manzi, our director of Technical Information & Services, Christen Sachse-Vasquez, and our Communications manager, Gary Sullivan, will join me at the booth. We’re excited to meet the people who create the fragrances we all enjoy so much. We see this as a unique opportunity for us to listen to perfumers’ concerns and to put a face to the science and resources that ensure every fragrance in every perfumer’s palette can be safely and confidently used.
 RIFM president Anne Marie Api, Ph.D.
RIFM president Anne Marie Api, Ph.D.
Anne Marie Api, Ph.D.
RIFM president Anne Marie Api, Ph.D., Fellow ATS, has been with RIFM for 40 years, directing the Institute’s extensive research programs in dermatotoxicology, genotoxicity, repeated dose toxicity, reproductive toxicity, environmental sciences, human exposure, respiratory toxicity, computational toxicology, and safety assessments.
Api is a co-author of the Quantitative Risk Assessment (QRA) for dermal sensitization, which guides the fragrance industry’s global standards and shepherds the groundbreaking Creme-RIFM Aggregate Exposure Model for fragrance ingredients. This model provides a realistic estimate of consumer exposure to fragrance ingredients in cosmetic, personal care, air care, and household care products that allows for risk assessment and management. In addition, she is a leader in expanding RIFM’s use of animal-alternative methods and models.
 Senior scientist Isabelle Lee, Ph.D.
Senior scientist Isabelle Lee, Ph.D.
Isabelle Lee, Ph.D.
Senior scientist Isabelle Lee, Ph.D., earned her Ph.D. in Pharmacology from the University of Pennsylvania, where she defended her dissertation on “The role of polycyclic aromatic hydrocarbon (PAH) metabolites in endometrial cancer.” After graduation in 2019, Lee started with RIFM as a post-doctoral researcher and now leads RIFM’s Skin Sensitization Safety Assessment and Research Programs.
Footnote
ahttps://doi.org/10.1016/j.yrtph.2024.105569










