For attendees of this year’s World Perfumery Congress, it was clear that consumer-driven trends in the global beauty market are impacting the fragrance industry. The large variety of natural materials being offered on the exhibition floor, as well as presentations focusing on consumer demands, demonstrated how the industry is evolving to meet current consumer preferences including:
- Use of non-petrochemistry-based ingredients, preferably all-natural and with corresponding certifications from independent agencies.
- transparent sourcing of these ingredients guaranteeing sustainability and minimal ecological impact.
- simpler formulas leading to multi-functional products with consumer-friendly labeling.
- ingredients that address cultural preferences and sensitivities.
For the fragrance industry, one way to accommodate these preferences is by replacing alcohol as the carrier solvent with biology-based hydrocarbons (BBHCs),a which allow for the design and manufacture of entirely novel multifunctional products that can carry an “all-natural” certification1.
Historical Context
For most of humanity’s scented history, the distinction between beauty care and perfume did not exist. Ancient cultures used fragrant ointments, unguents and oils to keep skin and hair soft, youthful and healthy, while off-setting ever present malodors. Many of the strong and alluring scents extracted from botanical and animal sources, often combining cosmetic and medicinal properties, are still used today and form the base of natural perfumery. Traditionally, these products - be it a massage oil in Cleopatra’s boudoir recalling the fragrance of blue lotus flowers on the Nile or a rose petal-scented lip paper at the imperial court of the Sung dynastyb resulted from extracting fragrance from biological materials by means of maceration in lipophilic bases such as vegetable oils, animal fats or beeswax.
Distillation as another technique to enrich natural fragrances has also been known for thousands of years2, but only with the systematic application of steam-distillation techniques by Indian and Spanish-Arab chemists in the 12th century C.E. has it led to new highly concentrated products called “essential oils.” Similarly, early attempts to distill the “essence” of wine led to the discovery of alcohol or Spiritus Vini, as the alchemists called it. While these early “spirits” were most likely a mixture of many different alcohols carrying a strong odor, refining of distillation technology led to the availability of relatively pure ethanol during the early Renaissance. Thus it is generally accepted that the first ethanol-based fragrances emerged about 600 years ago3,4.
The high volatility of ethanol, its low cost when produced in a petrochemical setting,c and its miscibility with water, allows for the creation of a plethora of fragrance products. This contributed to alcohol’s worldwide adoption as a fragrance carrier of choice, with the exception of countries and communities where its use is limited for cultural reasons. It is here where the ancient traditions of oil-based perfumery have blossomed, as visits to the perfume bazaars in Dubai, Marrakech, Dacca or Kuala Lumpur amply demonstrate.
A growing niche market already exists for non-alcoholic fragrances, such as the so-called huiles de parfum (for example “Si” by Giorgio Armani, or the various perfumed oils by the Swedish brand Byredo), not to mention the successful eau miscellaire designation, all of which have a dedicated following. However, these products use synthetic solvents or solubilizers in their formulas, which precludes them from being labeled “natural” for wider customer appeald.
With the introduction of cosmetic-grade medium-chain BBHCs as an alternative, nature-derived, non-alcoholic fragrance carrier, another chapter in the history of perfumery can be written.
Limitations of Alcohol and Vegetable Oil as Perfume Carriers
Oil-based fragrances have a certain appeal of having emollient and hydrating effects, due to oil’s film-forming abilities; in that sense, they are multi-functional products, combining fragrance and skincare properties. Alcohol, on the other hand, lacks cosmetic qualities. In fact, many consumers feel that ethanol has a drying, even irritating or stinging effect on their skin.e Added to this, there is growing concern about the detrimental effect of alcohol, with its pronounced anti-microbial activity, on the skin microbiome.f This stems from an emerging consciousness about the important role of diverse microorganisms in maintaining healthy skin5, and mounting scientific evidence that correlates such skin conditions as hypersensitivity, eczema, or rosacea with imbalances in the natural skin microflora6.
Vegetable oils and natural fats, while harmless towards the skin microbiome, have their own limitations as fragrance carriers due to their intrinsic chemical and physical characteristics. First and foremost, there is the issue of limited oxidative stability. Chemically, natural oils and fats are glycerin esters of mostly medium-chain fatty acids of eight to 18 carbon atoms (a.k.a. “triglycerides”; see F-1), and many also have one or more double bonds in their molecular structures. Exposed to air, these double bonds can react with oxygen, leading to often malodorous end products (rancidity) that can easily overpower the intended fragrance in a formula. Consequently, natural oil-based fragrances are best prepared fresh. Physically, oils and fats share high viscosities and very low volatility, which account for their tendency to leave residues on fabrics. In perfumery practice, that lack of volatility warrants special production technologies and generally translates into less radiance of oil-based scents, making them more intimate in comparison to alcoholic perfumes. And of course, oils as fragrance carriers create an oily skin-feel that may be disliked for esthetic or medical reasons, for example in cases of acne-prone skin.
Overcoming the Limitations of Traditional Perfume Carriers with BBHCs
Following the growing consumer demand for more natural cosmetic ingredients, medium-chain BBHCs were introduced a decade ago as “natural”7 replacements for the ubiquitous silicones (e.g cyclomethicones and dimethicones). New BBHC-based materials, including highly purified fractions with well-defined physical properties8, or sophisticated blends that match the properties of a particular silicone ingredient9, are increasingly becoming available. As such, their current spectrum of applications includes skin-emollients and make-up removers, sunscreens, and hair care products. Unlike oils, they are stable, largely odorless, and do not leave any residue or stain on skin or fabric. In contrast to alcohol, they do not have any negative effects on the skin. In fact, they share with silicones a particular silky skin feel that is highly prized among consumers. Employing BBHCs as carrier solvents, therefore, allows for the development of modern fragrances with the added and desirable qualities of a safe and versatile cosmetic product.
The Nature of BBHCs and Their Role in Skincare and Perfumery
Hydrocarbons that contain between eight and 20 carbon atoms, whether in a linear (abbreviated “normal-“ or “n-”) or branched arrangement (abbreviated “iso-” or “i-”), are called medium-chain alkanes (INCI).g If derived from manufacturing processes that mimic natural conversions, such as those shown in F-2, they can be called biology-based hydrocarbons (BBHCs). These are water-clear, colorless liquids of relatively low viscosity and distinctly lower vapor pressure than their short-chain relatives, n-hexane or petrol ether, or ethanol for that matter (T-1). As a result, these liquids take longer to evaporate at ambient temperature. n-Dodecane (linear C-12; F-2,1), for example, lasts 2.5 hours on a blotter at room temperature, while branched isomers, such as the various iso-dodecanes (F-2, 2) take considerably less timeh and a commercially successful blend of C-13 to C-19 straight-chain hydrocarbons lasts 3.5 hours. These properties are identical for petrochemistry- or biology-derived alkanes.
Medium-chain alkanes have been an integral part of skincare since the 19th century, especially in hydrating and emollient applications for sensitive skin, such as baby creams and lotions. Different blends, often proprietary, are generally known as “paraffin oil,” “mineral oil,” or petrolatum (INCI). Similar to the silicones, petrolatum cannot be certified as a natural ingredient, and suitable blends of BBHCs are increasingly being used as nature-derived replacements in many new “green” skin- and hair-care products. Fragrances based on BBHCs would naturally share these advantages, providing an enduring emollience that lasts over the lifetime of the scent. While longer-chain BBHCs are odorless, the shorter cosmetic-grade C-8 to C-13 congeners have a characteristic, albeit weak, slightly citrusy scent of their own, reminiscent of fresh laundry. This natural scent might be welcome in certain fragrance contexts such as aftershave or body spray applications, but can also be easily masked without great effort.
Compliance of BBHCs with Accepted Standards for “Naturalness”
The large-scale availability of biologically-derived alkanes for cosmetic purposes is actually a side effect of the global search for fossil fuel alternatives from plant or microbial sources because the primary production processes for bio-diesel are identical (F-2). The chemical reactions involved are simple chemical conversions of oils and fats, or biomass (e.g. sawdust, corn husks,), employing heat and pressure, mineral acids or bases, hydrogen gas, catalysts, or various micro-biological processes, without the addition of any potentially harmful reagents or petrochemicals that could be carried over into consumer products. Consequently, such conversions can be seen as an extension of natural transformations, and are thus allowed by organizations such as ECOCERT-COSMOS or NPA to be included in the manufacture of certified-natural and sustainable cosmetic ingredients7. As such certifications convey a distinct commercial product advantage, most BBHC ingredients carry them at this time.
The Role of Hydrocarbons in Traditional Fragrance Manufacturing
Odorant molecules are lipophilic in nature, meaning they prefer to interact with and dissolve in non-polar matrices such as oils and fats, or solvents such as hydrocarbons, certain esters, ethers or alcohols. Short-chain alkanes, such as hexane and petrol ether are widely used to extract fresh botanical materials at relatively low temperatures under conditions that keep the often chemically sensitive fragrant constituents intact. These highly volatile solvents are then evaporated and recycled, leaving fragrant waxy masses behind, the so-called concretes. Elaborate extraction and washing processes with ethanol, known as lavage, followed by low-temperature concentration yield the absolute, which until today comprises the highest fragrance concentration among natural perfume ingredients10. However, a fundamental problem with this processing sequence is the high flammability of the short-chain hydrocarbons and of ethanol as well. A sophisticated explosion-proof manufacturing environment is essential, which poses challenges to producers in terms of capital expenditure and guaranteed worker safety.
Extracting and Enriching Fragrance with Medium-chain Alkanes
Highly fragrant extracts can be obtained by exposing fresh botanicals, such as flowers or herbs directly to medium-chain alkanes. The resulting, usually lightly colored liquids are essentially liquid concretes or liquid pomades of 1:1 extracts with 90% C-13/C-15 and 10% diocytl carbonate (see F-3). The fragrance of these liquids, albeit lighter in character than either those of a concrete or absolute, captures the scent of the living flower extraordinarily well. This is especially true in the cases of jasmine and the white and yellow butterfly gingers, the latter being a resurrection of the scent of the legendary Longoza absolute from the Madagascan island of Nosy Be11. In the same way, crude botanical extracts such as supercriticalfluid extracts (SFEs) from liquified carbon dioxide12 or the concretes can be further extracted with medium-chain alkanes to give refined products called neo-absolutes. In contrast to traditional absolutes, the fragrance character of these products is much closer to that of the natural botanical source, especially for such classics as jasmine or tuberose, as no additional heat treatment for the removal of ethanol is involved. In this regard, these materials are modern versions of the classic lavagebefore it is concentrated down to the absolute, and which has always been considered as having the more true-to-nature scent compared to the latter13.
BBHCs can be employed in the manufacturing of natural fragrance materials in much the same way as their short chain relatives, and yet offer several important advantages. In contrast with the latter, the lower vapor pressures and volatilities of BBHCs render them essentially non-explosive under normal extraction conditions (T-1). At the industrial scale, this translates into increased work safety and lower capital expense. To offset the slightly lower extraction power of BBHCs relative to hexane or petrol ether, small amounts of polarity-modifiers can be added, such as volatile, nature-derived ethers or esters, that allow for fine-tuning of solvating selectivity towards fragrance components of particular interest or value. Several of such polarity modifiers are themselves cosmetic ingredients and are available with “green” certifications.In the author’s experience, dioctyl-ether or dioctyl-carbonate, among others, have proven to be very useful in this regard. With such solvent combinations, low-temperature single-batch extractions are very straightforward. However, dynamic extraction processes can be designed where successive batches of fresh plant material are exposed to a continuous flow of solvent, which becomes slowly enriched with fragrant components until a desired concentration is achieved. Two examples illustrate the simplicity of raw material extraction with medium-chain alkanes (see side panel).
In contrast to products from traditional extraction processes, BBHC extracts display excellent emollient and film-building properties when applied to the skin, due in large part to the presence of co-extracted waxes and fats,i plus the fact that the solvents themselves are excellent skincare emollients. As such, they can be directly used in downstream blending and formulating, often without the need for costly solvent removal and refining. Last but not least, these new products fulfill the requirements for “natural” certifications.j
Selective Fragrance Extraction and Concentration
For creators of novel multifunctional fragrance materials, the selective enrichment of scent molecules by medium-chain alkanes, while leaving behind most colored components and other unwelcome impurities, is a serendipitous quality. Figures F-4, F-5 and F-6 show BBHC extracts of a range of different raw materials. The images were taken before the separation of the BBHC extracts (upper phase) from the often deeply colored residues at the bottom of the vials. The undesirable components are usually more polar than the fragrant molecules and are co-extracted when using solvents of higher polarity, such as ethanol or supercritical-fluid carbon dioxide or solvents used in the preparation of classical concretes, namelyhexane and petrol ether. This leads to a dilution of the fragrant molecules by non-fragrant impurities. By eliminating such impurities, the medium-chain alkanes can result in a more concentrated fragrance, which has interesting economic implications for perfume production, as discussed further below.
The large variety of BBHCs enables the creation of custom solvent blends that can be fine-tuned towards the enrichment of particular fragrance components, either directly from a botanical matrix or from extracts, such as concretes, absolutesor SFEs. Used alone or in combination with other slightly more polar solvents from the gamut of natural skincare ingredients, medium-chain alkanes can produce quite original fragrance materials, comparable to the many “heart notes” that have become commercially available. Moreover, this is accomplished at a considerably lower production cost than fractional distillation.
The use of BBHCs to selectively extract fragrant molecules from a complex mixture can have the added benefit of removing harmful impurities such as allergens and irritants. To illustrate this point, F-7a shows the successful removal of allergenic aldehydes from a commercially available, IFRA-compliant oakmoss absolute by extraction with a BBHC. The H-NMR spectrum of the BBHC extract retains all dominant fragrance components, namely methyl-orcinol carboxylate (Evernyl®, Veramoss®, etc.) and its congeners14, (as evidenced by numerous signals between 6.15 and 6.35 ppm (F-7b), however, the traces of allergenic aldehydes present even in the IFRA-compliant absolute (as revealed by the sharp signal at 10.34 ppm) have been completely eliminated by BBHC extraction. Maybe there is new hope for the return of oakmoss to the perfumer’s palette, after all.
Unfortunately, this technique has its limitations. Cold-pressed bergamot essential oil contains compounds of the furo-coumarin type (also known as Psoralenes) that are potent photo-sensitizers, leading to sunlight-induced dermatitis. Consequently, such oils are restricted by IFRA to 0.4% in products that will end up on sun-exposed skin. Unlike the effect on oakmoss absolute, mixing the oil with 6 different BBHCs did not precipitate these compounds, as can be shown in a thin-layer chromatography analysis (F-8): the furo-coumarin Bergaptene shows a characteristic green fluorescence in the cold-pressed oil (traces 8&9), while other coumarins appear bright blue. The same pattern can be seen in all six BBHC extracts (traces 1-6), while a furo-coumarin-free (FCF) distilled oil does not contain any of these compounds (traces 7&10).
When a further concentration of fragrance molecules is desirable, it is possible to exploit the behavior and changing solvating powers of different BBHCs at lower temperatures to accomplish this by the selective precipitation of impurities. As T-1 shows, most linear medium-chain alkanes solidify at temperatures below 15ºC. However, careful blending, sometimes with the addition of branched-chain isomers, yields mixtures that remain liquid down to temperatures of -20ºC and below (see F-9). Many impurities in a fragrance will become insoluble at low temperatures and precipitate, which makes their removal straightforward. Compared to costly vacuum distillation, the selective precipitation of impurities offers an inexpensive path to purify and concentrate fragrance components, either from natural matrices (e.g., flowers, herbs, spices, resins, etc.) or from crude extracts of these materials such as SFEs or concretes. As an example, F-10 shows the treatment of an SFE of coconut meat with three different BBHCs at -18ºC. While all three solvents are still liquid at this temperature, a commercially available blend of C-13 to C-19 hydrocarbons (left vial) best excludes the large amount of non-fragrant coconut fat which was present in the original extract but has now precipitated out. Consequently, the supernatant in the left vial contains the fragrant components in their highest concentration; once separated from the precipitate by cold-filtration or aspiration, it smells like freshly baked macaroons.
Improved Chemical Stability of Medium-chain Alkane-based Fragrances
Traditional alcohol-based perfumery practice demands a maturation period for newly prepared extraits, eau de toilettes, or eau de colognes, often of several months’ duration at low temperature, followed by cold filtration13. During this period, chemical transformations occur as the ethanol reacts slowly with components of the fragrance concentrate, resulting in a more integrated fragrance sensation often described as “smoothing the edges”13,15. In contrast, BBHC-based perfumes do not require or benefit from prolonged cold storage, as the carrier is chemically inert and thus not amenable to reactions with the scent molecules in the concentrate. A brief 24-hour chilling of BBHC-based fragrance concentrates at a temperature just above the crystallization temperature of the alkane blend, followed by filtration or centrifugation, is all that is needed to obtain stable products which will not change their fragrance impression over time.
A further characteristic of BBHC-based preparations is that dilution with more of the same alkane or alkane blend results in a more dilute but otherwise unaltered fragrance. This contrasts with alcoholic scents that are brought to their final concentration through dilution with water, where the diminished solubility of crucial scent components at lower alcohol concentration can lead to a different scent impression. In contrast, an eau de cologne-equivalent in BBHCs will have precisely the same, albeit weaker, scent as a more concentrated extrait.
F-10 illustrates the gain in simplicity and efficiency of extractions with BBHCs in direct comparison to traditional process sequences:
- Extraction of raw natural materials yields lightly colored fragrance materials called neo-pomades, because the process resembles traditional enfleurage; these liquid extracts can be directly incorporated into an “all-natural” cosmetic product, or further blended into a scent composition.
- Further extraction of traditionally produced materials such as concretes, absolutes, SFEs, or even essential oils leads to highly enriched fragrance materials that are distinguished by the almost complete absence of colored or otherwise unwelcome impurities; these materials are called neo-absolutes.k
As noted above, the artful blending of different BBHCs, with or without the addition of “green” polarity modifiers, allows for the selective enrichment of certain scent components, giving novel olfactory characteristics to the resulting extracts. For the manufacturers of natural fragrance materials, as well as for the creative perfumer, this opens new pathways towards proprietary all-natural scents and multi-functional beauty products with top note characteristics rivaling those of synthetic blends.
Direct Comparison of an Alcoholic and an Alkane-based Perfume
Interestingly, the overall fragrance characteristics of BBHC extracts differ, albeit in subtle ways, from alcoholic extracts. The BBHC-based neo-absolutes seem to be more radiant, emphasizing top notes over base notes, whereas traditional alcohol-based absolutes – once the carrier has evaporated – seem to convey a slightly muted, more complex and longer lasting scent impression.
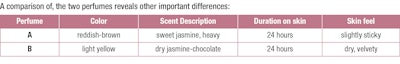
To illustrate the differing characteristics of BBHC- and alcohol-based perfumes, F-11 compares a traditional alcohol-based perfume at 20% fragrance concentration (A) with the corresponding alkane-based perfume (B) at the same 20% concentration.l Both were prepared from the same scent concentrate that was diluted to 20% by addition of ethanol or the alkane blend. After over-night chilling the alcohol-based product is deeply colored and turbid, while the hydrocarbon solution is clear and only lightly colored; all ballast materials have collected at the bottom of the vial, making a final separation straightforward and inexpensive.m
The alcoholic version A had all the fragrance characteristics of a traditional “French” jasmine perfume with a sweet fruity-flowery top note, albeit on the heavy side. In comparison, the non-alcoholic perfume B exhibited a stronger yet harmonious chocolate-jasmine aroma that can be described as dryer, less fruity than A and overall lighter and more “contemporary.” Sprayed onto skin with an atomizer, the alkane blend spread easily and with a comfortable warming sensation. After less than a minute there was no residue, save for the strong yet very elegant fragrance. After five minutes on the skin, both fragrances developed a citrusy amber note, which evolved into a very feminine muskiness after an additional 30 minutes. After 3, 12 and - in case of A - 24 hours, the jasmine-musk theme was still clearly dominant with the chocolate notes becoming increasingly noticeable in B.
Six female volunteers were asked to compare the two fragrances in terms of 13 sensory characteristics. The results, plotted in “spider graph” format, are shown in F-12. Most interestingly, the non-alcoholic version B was rated unanimously as much too strong compared to the alcoholic version A. Only after further diluting the BBHC blend to 7.5% did the panel feel a strength equivalence to the alcoholic version (at 20%). Thus it could be demonstrated that the hydrocarbon-based perfume had changed the original scent profile towards a brighter onset that warranted further dilution, and yet upon skin application behaved just like a classical alcoholic fragrance in the sense of olfactory progression from top- through heart-note and finally dry-down, and in a development time frame that is well within the range for any high-end perfume. When version B was incorporated into an alkane-based body mist (formulated as a water-in-oil emulsion), a final concentration of only 0.2% (w/w) was sufficient to give the desired signature scent, whereas concentrations of around 1% which are more typical for this type of product were deemed too strong.
At this point, a satisfying explanation for the differing scent impressions created by the two preparations is not available. In practical terms, however, the higher sensorial impact of alkane-based fragrance compositions points towards a possible economic advantage, as the concentration of sometimes very expensive natural ingredients can be lowered without compromising the olfactory effect.
Alcoholic perfumes, on the other hand, persist on the skin for a longer time than alkane-based compositions (about 40% longer in the case of A vs. B). This may be due to the co-extraction by ethanol of more polar or higher molecular weight components of the initial fragrance concentrate that possess fixative properties. When desired, the fixation of BBHC perfumes can be achieved by the addition of stable, nature-derived, high-molecular-weight components that are soluble in the hydrocarbon solvent base, colorless and odorless, safe in a cosmetic context, and do not alter the skin feel. As an example, a 1% addition of the rapeseed-oil derived capryloylglycerin /sebacic acid copolymer in diheptyl succinate (INCI)16 extends the lifetime of B on the skin by about 20% without flattening the scent or otherwise negatively impacting the application.
Standardized Fragrance Building Blocks and Automated Perfume Creation
In contrast to ethanol, use of medium-chain alkanes in the preparation of neo-pomades or neo-absolutes enables the perfumer to work with stable pre-formulated solutions of fragrance materials standardized to a given concentration. Such solutions can be considered “fragrance building blocks.” This apparently simple quality paves the way for an entirely new platform technology for future perfumery practice. As we have seen, ethanol’s volatility and chemical reactivity renders it unsuitable for preparation of standardized solutions. On the other hand, medium-chain alkanes, being chemically inert and of low volatility, allow for the creation of stable “fragrance libraries,” that do not evaporate or change in character over time. Such libraries can contain hundreds of standardized fragrance materials, all having the same concentration, density, viscosity, and low coloration as the examples described above. They can be organized according to common scent notes, such as woody or floral, or by characteristics such as lactonic or sandalwood-like, completely at the perfumer’s discretion. Moreover, such libraries can be stored in specialized containers (multi-well plates), for automation of the blending process.
The blending of BBHC-based fragrance building blocks prepared at a predetermined concentration of, let’s say, 20% for an extrait, allows the perfumer to follow the development of a formula inreal time.During the creative blending process, while the ratios of the individual components differ, the concentration of their sum in the increasingly complex blend stays always the same as that of the end product. Unlike working with a fragrance concentrate, there will be no necessity for dilution and maturation at the end of the process that may alter the overall olfactory profile. Consequently, an idea of what the final composition will smell like, and whether or not this conforms with the requirements of the brief, emerges from the first blending sequence onwards. Not only does this simplify the creative process, but it also opens up the possibility for its automation (see F-13).
The consistent physical and chemical characteristics of a BBHC-based fragrance building block allow for thea-priorisetting of liquid handling parameters such as pipetting volumes, weighing and blending, which will not change over the duration of the experiment. Liquid handling robots17, now a standard feature in any pharmaceutical drug discovery laboratory, are machines that automatically dispense precise, predefined amounts of reagent liquids into containers, usually at a very small scale. The standard format is the so-called multi-well or microtiter plate where the vials are actually small containers, called wells, fused to each other and arrayed on a 8 x12 = 96-well grid of fixed geometryn (see F-14).
In a scientific, e.g. pharmaceutical setting, these technology platforms share the epithet “high throughput,” as in “high-throughput screening.” This means that hundreds, sometimes thousands of data points can be gathered overnight, and analyzed by scientists the next day. In a perfumery context, building blocks with particular scent profiles, for example mossy, bergamot and patchouli, can be blended together in varying ratios, to yield hundreds of chypre accords in one night, awaiting olfactory evaluation by the perfumer the next morning. The perfumer’s creative mind only specified the building blocks and how to mix them, allowing the machine to do the rest. The machine can be further programmed to transfer a small amount of each final blend to the corresponding grid position on a sheet of blotting paper, which becomes a two-dimensional mouillette (see F-15)for evaluation by nose.o
An example of blending on a 96-well grid is depicted in F-16. The client brief called for a pre- and after-shave gliding mist with anti-inflammatory activity and a “trendy” scent with a culinary twist. A 96-well plate was filled with a constant amount of a fougere blend (white square) and overlaid with three building blocks containing two single standardized BBHC extracts (yellow and magenta) and one anti-inflammatory blend (blue). The latter were applied orthogonally (i.e. left-to-right, up-to-down, right-to-left) in serial dilution, yielding 96 different compositions. After evaluating the individual accords, the “twist,” a standardized blend of coffee bean SFE and Splendione®, was added to some of the wells in the center of the plate. If automated, this process would produce a novel fragrance base in only two days, upon which a final formula could be elaborated. The entire project would be finished after two weeks.
The blending of scents with the goal of creating a new perfume is a combinatorial process that can be described in precise mathematical terms. Interestingly, a seminal treatise on combinatorial mathematics by the Indian scholar Varahamihira, written around 550 C.E., uses the combination of 16 basic scents to create four perfumes out of 1,820 possibilities as an example for a combinatorial matrix or prastara18. Such mathematics can form the basis for machine learning algorithms that can instruct a liquid handling robot to independently blend fragrance building blocks into entirely novel accords19. The perfumer’s creativity and professional knowledge will then determine which accord to proceed with in accordance with pre-determined criteria such as a particular brief, consumer trends, or simply novelty. Thus, BBHCs as fragrance carrier solvents open an entirely new way for olfactory creation, one where the perfumer can work in true partnership with an artificial intelligence.
The Remaining Questions: Sustainability and Material Costs
Most BBHCs are currently produced from plant oils and fats with the exception of hemisqualane, which is a product of microbial biotechnology. The main source crops are oil palm (Elaeis guineensis) and coconut palm (Cocos nucifera), with world-wide plantations covering more than 27 million hectares. Because of the climatic requirements of these two crops, vast areas of tropical rainforest have been converted into industrialized plantations, destroying unique and irreplaceable biospheres and natural habitats. As noted at the beginning, the emerging consumer consciousness on issues such as naturalness of ingredients and their sustainable production is creating increased pressure on the cosmetic industries to use production methods that conform to the ideals of ecologically sound practices20. Most producers of BBHCs have therefore adjusted their procurement strategies in recent years to comply with internationally accepted standards for natural cosmetic ingredients and the corresponding production processes, such as those promulgated by ECOCERT/COSMOS in Europe or the Natural Products Association (NPA) in the U.S. Alternatives to palm or coconut oil are already being pursued, utilizing rape seed (Brassica napus) or castor oil (Rizinus communis) as source materials with the advantage that these plants grow in temperate climate regions and share a long history in Western culture. The improved carbon footprint of such home-grown products will add to their commercial attractiveness. Ultimately, however, even sustainable agriculture is no match for microbiological processes, which is why bio-diesel is increasingly being produced from the oil of micro-algae, with edible oil production in close pursuit. It is this development that allows for optimism about the commercial future of BBHCs, as they follow the same production schemes as bio-fuels, but with guaranteed sustainability21.
Large-scale production of BBHCs in an agricultural or microbiology setting that is geographically closer to market will ultimately also bring the costs of these ingredients down to attractive levels. BBHCs purchased in bulk cost presently between $20 and $30 per kilogram, while petrol-derived cosmetic-grade iso-dodecane, for example, runs at about $10. Unlike hexane and alcohol, BBHCs are not being recycled in the context of multifunctional perfumery. This means that cost savings will have to be realized from less expensive production processes, lower capital expenditures, and possibly lower ingredient costs, stemming from the higher olfactory impact of the finished products which allows for lower concentrations to be used. Finally, breaking from the fragrance into the wider beauty care market with multi-functional products will ultimately lower production costs and increase revenue following the laws of economy-of-scale.
Conclusions
Substituting alcohol in a fragrance product with cosmetic-grade medium-chain alkane liquids, preferably of biological origin (BBHCs), enables the creation of products that substantially differ from traditionally produced perfumes in terms of:
- new fragrance profiles from classical starting materials,
- minimal color and non-staining,
- cosmetic applications with superior skin-feel (non-alcoholic, non-oily, non-stinging, non-irritant),
- improved production economy due to lower process safety requirements, and the selective enrichment of fragrant molecules in the end product,
- chemical stability and substantially increased shelf-life in comparison to traditional oil-based fragrances,
- the potential for certification as “natural,” since the carriers can be of plant origin and sustainably produced, such as the BBHCs introduced here.
Furthermore, the chemical and physical characteristics of medium-chain alkanes allow for the implementation of automated blending platforms with the potential to revolutionize the creative perfumery process.