A compounded flavor is a complex blend of chemical substances chosen to give a desirable flavor effect. The most common form of delivering compounded flavorings is in the liquid form; essentially the desired blend of aroma chemicals dissolved in an organic solvent. The flavor solvent is most commonly ethanol, propylene glycol (PG), benzyl alcohol, or medium chain triglycerides (MCT). While the blend of aroma compounds dissolved in the solvent is dependent upon the flavor being reproduced, one would expect to find a wide variety of chemical functional groups e.g., hydroxyl, carbonyl, carboxyl, benzyl, etc.1, 2.
Thus, it should be no surprise that chemical reactions are likely to occur between the aroma chemicals as well as between the aroma chemicals and the solvent system. This means that the resultant flavor is likely to change over time due to these chemical reactions3. As a result, compounded flavorings have an expected shelf-life and should be discarded beyond that shelf-life. Developing a food product based on the use of an out of date flavoring can be disastrous in that it may be impossible to reproduce that flavor if desired.
Carbonyls are key compounds to many flavors and odors4. They may be simple aliphatic (C1-C12), unsaturated, benzylic, or phenolic type structures. Condensation of a carbonyl with an alcohol (for example ethanol or propylene glycol) readily takes place in the presence of an acid catalyst resulting in formation of an acetal. Acetal formation changes the flavor by removing the carbonyl compound and the alcohol. Since alcohol is greatly in excess as the solvent, its loss is largely irrelevant. However the loss of the carbonyl compound in the formation of an often obnoxious smelling acetal may have a very negative effect upon sensory perception.
Acetal formation has been observed in commercial beef flavorings5 and cherry flavor1. Since the formation of an acetal is an equilibrium phenomenon catalyzed by acids, acetal hydrolysis may occur resulting in regeneration of carbonyls upon dilution of acetal in acid conditions (e.g. in beverages). Heydanek and Min reported that adding 0.5% cherry flavor containing acetals to a soft drink solution containing 11% sucrose and 0.8% citric acid showed no traces of acetal1. The authors did not provide any details of the hydrolysis conditions to regenerate free aldehydes, but they commented that total hydrolysis may not occur if the medium is at neutral pH or without moderate heat processing. Considering the importance of acetal formation on flavor quality, there is a need to better understand the factors controlling acetal formation in commercial flavorings, e.g., flavor solvent, type of carbonyls, type of alcohol, presence of water and acids.
Two related studies by Leclercq et al.6, 7 have added some insights on aroma compound stability in liquid systems consisting of various oil matrices (MCT, sunflower oil, or soybean oil) and water under different storage environments (ambient air or argon atmosphere). The authors found that model compounds were considerably more stable when stored in an oil-based system than an aqueous system and MCT offered substantially greater stability to the volatile compounds than sunflower oil or soybean oil. Moreover, as one would expect, the model volatiles were typically less stable in an ambient oxygen level environment than a low-oxygen environment regardless of the matrix system they are diluted in.
The present study has three main objectives:
- investigate the stability of aroma compounds with different functional groups in model flavor mixtures. Our interest is to evaluate the chemical reactions that can occur in compounded flavorings – when we talk about a model flavor mixture, that doesn’t necessarily mean that it’s formulated like a commercial flavoring, however, we specifically chose model systems that mimic a commercial flavoring;
- evaluate factors controlling acetal formation; and
- identify aroma deterioration mechanisms in model mixtures.
The primary goal of this paper is to raise the awareness of potential interactions among flavor solvents and aroma compounds which result in a flavoring changing over time even when it is stored as a liquid. Moreover, it provides insights on how to minimize flavor deterioration.
Materials and methods
Materials
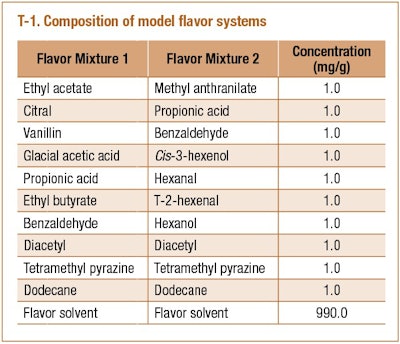
Two model flavors (Mixture 1 and 2) were prepared as described in T-1. These aroma compounds are representative of different chemical classes: alcohol, aldehyde, ester, acid, and heterocyclic aromatic organic compounds. Dodecane was used as an internal standard added to the flavor solvent. All chemicals were purchased from Sigma-Aldrich at the highest purity available (purity > 98% except citral and diacetyl with purity > 95%) and used at equal mass concentration (1 mg/g) in different flavor solvents. These compounds were selected in order to investigate flavor deterioration by formation of esters, acetals and oxidized products. The main difference between Mixture 1 and 2 is the presence of alcohols in Mixture 2 in order to evaluate reactions between carbonyls and alcohols.
Four solvents were used as flavor carrier: medium chain triglycerides (MCT), anhydrous ethanol (EtOH), 95% ethanol (WEtOH), and propylene glycol (PG). MCT (Miglyol®812) was purchased from Sasol Germany GmbH (Witten, Germany); other solvents were purchased from Sigma-Aldrich (Allentown, PA).
Methods
Preparation of flavor mixtures
Flavor mixtures were prepared according to the formulations presented in T-1. After finishing weighing all compounds, the mixture was vortex for 5s to ensure thorough mixing. In order to investigate acidification on flavor deterioration, 50 µL of 6 M hydrochloric acid was added into 10 g of flavor Mixture 1 using a micropipette (about 0.03 mmol HCl/g of mixture).
Storage study
The freshly prepared model flavor mixtures were made in 20 mL clear glass vials, and sealed with PTFE faced 14B rubber lined cap from Thomas Scientific Each flavor mixture was prepared in triplicate. Samples were stored standing upright in a dark incubator at 35 °C. Samples were taken periodically during storage for gas chromatographic-mass spectrometric (GC-MS) analysis.
Quantification of model aroma compounds
One µL of each sample was injected into a HP 5890 series II GC equipped with DB-Wax column (20 m × 0.1 mm × 0.2 µm, J&W Scientific). The operating conditions were as follows: head pressure, 12 psi; helium, 1mL/min; oven program, increased from 50 °C to 200 °C at 10 °C/min and then held at 200 °C for 5 min; injection port, 200 °C. A mass spectrometer (Hewlett-Packard model 5972 mass selective detector) equipped with Hewlett-Packard ChemStation software was used as detector. The parameters were set with scan mode of ions 29–450 and 1.84 scan/s. A Wiley library was used for tentative identification.
Concentrations of individual model aroma compounds (mg/g) in each mixture were determined via regression lines (R2 > 0.97) obtained from 5-point calibration curves. Dodecane added in the flavor solvent was used as internal standard for all aroma compounds. The amount of individual compounds remaining during storage was expressed as a percentage of their initial amount (day zero) in the mixture. Citral was reported as the sum of both isomers (neral and geranial). Each sample was analyzed in duplicate.
Quantification of reaction products
Reaction products were identified by matching with Wiley’s MS libraries. Concentrations of reaction products (e.g. acetals, esters, and acids) were expressed as the ratio of peak areas between reaction product and dodecane (1.0 mg/g). Each sample was analyzed in duplicate.
Statistical analysis
All samples were prepared in triplicate for analysis. Two measurements were conducted for each sample. The average was reported and standard deviation was calculated using Excel.
Results and Discussions
Flavor compound stability during storage
Flavor compounds in Mixtures 1 and 2 were selected to include a wide range of chemical classes typically used in flavor creation (e.g. alcohols, aldehydes, ketones, organic acids, and esters4, 8. Reactions between these chemicals and flavor solvents are the primary interest of the present study. To give an idea of the extent of reactions that might take place, the amounts of selected aroma compounds remaining in Mixture 1 after storage of 36 days at 35°C are presented in T-2.
Tetramethyl pyrazine was stable with almost 100% retention. Ethyl acetate and ethyl butyrate decreased slightly indicating possible hydrolysis. Propionic acid slightly decreased in all solvents which could be the result of ester formation with ethanol. In contrast to propionic acid, acetic acid slightly increased in all solvents. It is likely that ethyl acetate was partially hydrolyzed which would generate acetic acid. The conversion of acids and alcohols to esters and the hydrolysis of esters are reversible under certain conditions. The equilibrium can be shifted in either direction depending on the concentrations of individual substances and presence of catalyst (i.e. strong acids).
From T-2 one can also see that the carbonyl compounds (diacetyl and vanillin) were lost in all flavor solvents. Almost 90% of the vanillin was lost in PG indicating strong reactivity of the phenolic aldehyde in PG. Compared to vanillin, diacetyl, a diketone, was much less reactive in PG. The reactivity of different carbonyls and formation of acetals will be discussed in more detail in following sections.
Effect of Flavor Solvent on Acetal Formation
It is well known that carbonyls react with alcohols forming acetals. F-1 shows the amounts of benzaldehyde (benzylic aldehyde), and citral (α, β-unsaturated aldehyde) remaining during storage (Mixture 1). It is obvious that MCT has the highest retention of carbonyls, whereas propylene glycol has the lowest retention. A slight loss of citral in MCT was observed probably due to acid catalyzed cyclization and oxidative degradation3.
The substantial loss of benzaldehyde and citral in ethanol and propylene glycol is attributed to acetal formation. F-2 shows the formation of benzaldehyde acetals and citral acetals in anhydrous ethanol and PG during storage. Clearly, the formation of citral acetal is much faster than that of benzaldehyde acetal in both PG and ethanol; citral acetals reached a maximum after only two days of storage. Comparing F-1 and F-2, one can see that the loss of benzaldehyde and citral followed the trend of increased formation of the corresponding acetals. This clearly demonstrates that acetal formation is the main mechanism of the loss of benzaldehyde and citral during storage.
The presence of acetals could lead to quality issues depending on the flavor applications. In general, acidic conditions and heat favor hydrolysis of acetal and regeneration of free aldehydes. Heydanek and Min demonstrated that acetals in cherry flavor were completely converted back to the corresponding aldehydes when diluted in a soft drink type solution containing 11% sucrose and 0.8% citric acid1. The authors warned that in applications at neutral pH or lacking heat processing, total hydrolysis of acetal may not occur and batch-to-batch variation of flavoring in a product can occur. In another study conducted by Sharma and co-workers4, regeneration of citral from the corresponding dimethyl and PG acetals under mild acidic conditions showed that dimethyl acetal hydrolyzed to an extent of 85% whereas PG acetal formed a 1:1 equilibrium mixture with the generated citral. Therefore, special concern has to be taken when application conditions do not favor full hydrolysis of the acetal back to the free aldehyde.
Effect of Water in Ethanol Solvent on Acetal Formation
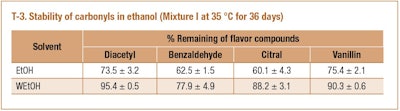
We would expect the presence of water to reduce the rate of acetal formation in a flavor mixture. Therefore, anhydrous ethanol and 95% ethanol were compared as flavor solvents for our flavor mixtures. As expected the results (T-3) showed that carbonyls have greater losses in anhydrous ethanol than that in 95% ethanol. As an example, the trends of benzaldehyde loss and formation of the corresponding acetal in both solvents correlated very well (F-3).
The presence of water in 95% ethanol has two effects. The process of acetal formation is governed by equilibrium. For many simple carbonyls, the equilibrium favors formation of acetal, but is inhibited by water9. In other words, by removing water as it is formed, the reaction can be forced to completion. Another effect of water presence is the dilution of acids (concentration of H+). Acids serve as catalysis in acetal formation which protonate the carbonyl. Therefore, the higher concentration of H+ in anhydrous ethanol resulted in greater conversion of acetal. A similar observation was reported in a system containing PG, aldehyde, water and citric acid [1]. The addition of citric acid greatly accelerated the formation of PG acetal. The impact of acid on acetal formation is further discussed in the following section.
The inhibitory effect of water on acetal formation may have practical implications in releasing aroma carbonyls. Most applications of liquid flavors are in acidic aqueous systems (e.g. beverages) where water is abundant. Upon dilution of flavor in water, acetal hydrolysis likely occurs resulting in regeneration of carbonyls.
Effect of Acidification on Carbonyl Stability
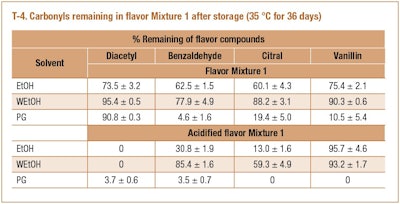
Flavor Mixture 1 was acidified by adding hydrochloric acid to the different solvents (0.03 mmol HCl/g mixture). Since acetal formation is an acid catalyzed reaction, one would expect a greater loss of carbonyls when acids are present in the mixture. T-4 shows the amount of carbonyls remaining in acidified solvents after storage (36 days at 35 °C). As expected, greater amounts of acetals were formed in acidified solvents. In acidified PG, all carbonyls were lost indicating greater reactivity of PG than ethanol. However, benzaldehyde had greater losses in 95% ethanol than in acidified 95% ethanol (22.1% vs. 14.6%) and vanillin showed greater losses in anhydrous ethanol than in acidified anhydrous ethanol (24.6% vs. 4.3%). These two exceptions were not understood. It is possible that vanillin has multiple functional groups including aldehyde, hydroxyl, ether and benzyl which make the reaction kinetics of acetal formation more complex than a simple alkanal. Among the four carbonyls, diacetyl showed the highest reactivity in all acidified solvents probably due to the α di-carbonyls being strong nucleophilies.
Since the formation of acetals is an equilibrium phenomena catalyzed by acids but not base, acetal formation can be inhibited or retarded by the use of neutral or slightly basic conditions. A patent on stabilizing carbonyl-based flavorings under basic pHs using NaHCO3 has been issued10. On the other hand, carbonyls can be stabilized as acetals under acidic conditions to prevent their deterioration and then potentially be released upon application (i.e. dilute in acidic aqueous phase). Sharma and co-workers evaluated formation of dimethyl and PG acetals and regeneration of free aldehydes (acetaldehyde, 3-hexenal, nonanal, citral, and cimmamic aldehyde)4. It was found that high yield of citral PG acetal (78%) could be achieved from the reaction of citral and PG. But free citral was recovered at only 50% from hydrolysis of citral PG acetal under specified conditions (1N HCl : aceton at 1:4 mL/mL). Interestingly, the authors found that citral dimethyl acetal had a higher conversion (85%) to free citral under the same hydrolysis conditions.
F-4 shows the loss of citral and formation of citral PG acetal in acidified PG during storage. Clearly, citral was lost dramatically in the first two days and completely disappeared after 16 days of storage. However, the concentration of citral PG acetal reached a maximum after two days and then decreased overtime. The loss of citral was not accounted for by the formation of the citral acetal. Since citral is extremely unstable in an acidic, aqueous system due to degradation and oxidation11, 12, it is possible that citral PG acetal was further reacted to other products.
Formation of Esters in Acidified Flavor Mixture 1
As discussed earlier, acetic acid is relatively stable in a flavor Mixture 1 (T-1). However, in acidified flavor Mixture 1, acetic acid decreased over time in all solvents and the greatest loss of acetic acid occurred in anhydrous ethanol (F-5). In the anhydrous EtOH system, acetic acid disappeared very quickly and the formation of ethyl acetate appeared to account for this loss. This result suggests that acetic acid reacted with ethanol forming ethyl acetate in acidified solvents. It is interesting that in acidified WEtOH, ethyl acetate increased steadily during storage, whereas acetic acid concentration was stable after 16 days of storage. Moreover, quantitatively the formation of ethyl acetate was greater than 100% conversion of acetic acid. This suggests that ethyl acetate was also formed from another pathway or acetic acid was formed during storage. Acetic acid was gradually lost in the PG system but there was no formation of ethyl acetate.
Surprisingly, acetic acid was detected in flavor Mixture 2 in which no acetic acid was added (F-6). Acetic acid was produced during storage in all solvents with the highest production rate in EtOH and the lowest in PG. It is likely that acetic acid was formed from oxidation and fragmentation of the carbonyls. One would expect acetic acid might also form in flavor Mixture 1, but we could not differentiate acetic acid originally in the mixture from that formed by oxidative reactions. If this is the case, we may able to explain the formation of the large quantity of ethyl acetate in acidified flavor Mixture 1. Further work is needed in order to elucidate the pathway of acetic acid formation.
Reactivity of Alcohols
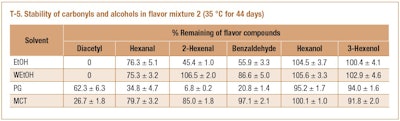
Based on results of aroma compound stability in Mixture 1, it was concluded that acetal formation was the main reaction mechanism of carbonyl loss. More specifically, carbonyls react with flavor solvents (ethanol and PG) and the acidification of flavor solvents accelerates acetal formation. However, the reactivity of flavor alcohols with carbonyls in the solvent systems has not been reported. Therefore, hexanol and 3-hexenol were included in flavor Mixture 2 to determine if these two alcohols participate in acetal formation. T-5 shows that all carbonyls (diacetyl, hexanal, 2-hexenal, and benzaldehyde) were lost to different extents in all solvents during storage. The carbonyls were primarily converted to the corresponding acetals (data not shown). However, both hexanol and 3-hexenol were relatively stable during storage indicating that they did not react with aldehdyes.
F-7 presents an example of the formation of different acetals in anhydrous ethanol. All these acetals were formed from the reaction of aldehydes with ethanol, not hexanol or 3-hexenol. This was probably because these two alcohols were present in small quantities (0.1%) whereas EtOH and PG as solvents were in abundant in the flavor mixtures. EtOH and PG were preferred to react with aldehydes to shift the equilibrium toward acetal formation.
Oxidation of Aldehydes
During storage in this study, all liquid flavor mixtures were stored in sealed glass vials in a dark environment. Since the glass vials were not completely filled (~ 8 cm3 of headspace), it was of interest to monitor the potential oxidation products. Two acids derived from aldehydes were identified. Hexanoic acid (from hexanal) and 2-hexenoic acid (from hexenal) were formed slowly during storage (F-8) which clearly demonstrated that oxidation could be a factor contributing to the loss of aldehydes. While the rates of forming these acids were slow, long storage times could change the aroma profile of the mixture.
Conclusions
The results of this study demonstrate that a formulated flavor is likely to undergo significant chemical reactions in storage: major reactions being acetal formation and oxidation. Acetal formation is an acid catalyzed equilibrium phenomenon which can be manipulated by flavor solvent, presence of water, and pH. The choice of flavor solvent has a large impact on aroma compound stability. If one were free to use any solvent in flavor creation, an unreactive solvent, i.e. medium chain triglycerides, would be a good choice. Ideally, PG and acids would be avoided in formulating flavors containing reactive carbonyls, e.g., vanillin, citral and benzaldehyde. When ethanol is used as a flavor solvent, the addition of a small amount of water can effectively reduce acetal formation.
Unfortunately solvent choice is typically dictated by final food application (water or oil continuous phase) and the solubility of the flavor components themselves (polar and nonpolar solvent system). Thus, it is worthwhile knowing what chemical reactions might occur and how quickly they will occur. It is helpful to understand that some reactions are reversible, for example acetal formation, which can make the flavorings smell very undesirable but might be totally acceptable on application since reaction is reversible. Recognizing some reactions are not reversible, food product developers should be taught that flavors deteriorate with time so they should not use an old flavor sample on his/her shelf but get a fresh flavor before beginning any product development.