Editor’s Note: Part two of this two-part column compares the chemical compositions of turmeric oil and Curcuma longa.
Turmeric Oil, Part 2
In addition, trace amounts (< 0.05%) of 2-hexanol, 3-buten-2-ol, (Z)-3-hexenol, 2-heptanone, 2-heptanol, α-thujene, camphene, a furanoid form of cis-linalool oxide, 2-decanol, perilla ketone, linalyl acetate, thymol, a sabinyl acetate isomer, tetradecane and cinnamyl cinnamate were found in this oil.
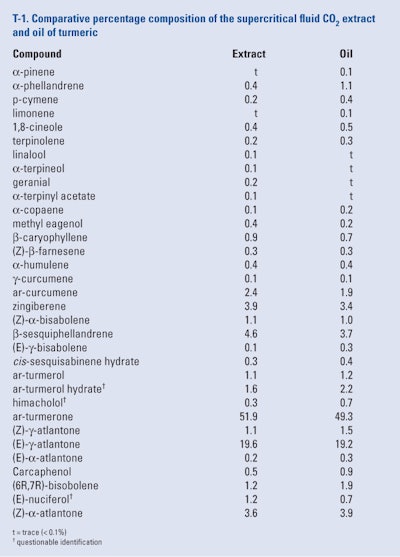
Marongiu et al. (2002) produced a supercritical fluid CO2 extract of turmeric rhizomes (available commercially in Italy) at 50°C, 90 bar and a CO2 flow of 1.5kg/hr. In addition, the authors also produced an oil from the same batch of rhizomes by hydrodistillation. The analytical results of the extract and oil, as determined by GC/MS only, can be seen in T-1.
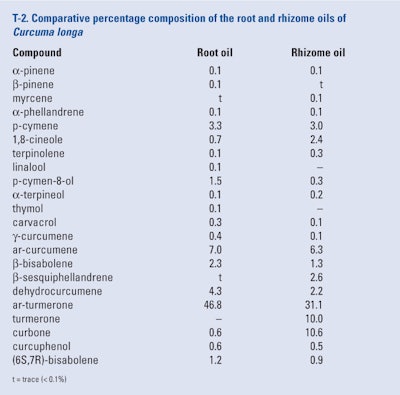
Leela et al. (2002) determined that the root and rhizome oil were produced in 4.3% and 3.8%, respectively, from C. longa grown on the experimental farm of the Indian Spices Research (Calicut, Kerala). The comparative composition of these two oils as determined by GC-FID and GC/MS can be seen in T-2. Trace amounts (< 0.1%) of β-pinene, β-phellandrene, p-methylacetopheneone, zingiberene and b-sesquiphellandrene also were found in one or both oils.
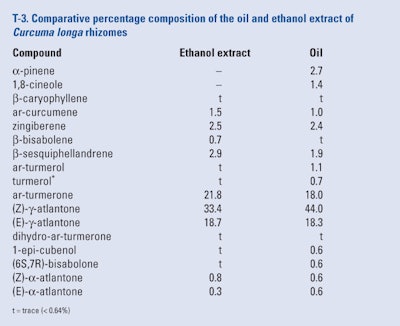
Braga et al. (2003) examined the yield and composition of supercritical fluid extracts produced using various conditions, low pressure solvent extract and a hydrodistilled oil of C. longa rhizomes grown in Minas Gerais (Brazil). The compositions of the low pressure ethanol extraction and the hydrodistilled oil were analyzed using GC-FID; these results and the retention time data are shown in T-3.
The analysis of a lab-distilled oil of turmeric rhizomes of Indian origin was determined by Singh et al. (2003) using GC/MS only; the researchers found the following major constituents:
α-caryophyllene (5.6%)
ar-curcumene (3.8%)
zingiberene (10.2%)
α-farnesene* (3.7%)
α-bisabolene (10.7%)
ar-turmerone (51.7%)
ar-turmerol†(11.9%)
*correct isomer not identified
†questionable identify
A commercial oil of turmeric root screened for its biological activities by Sacchetti et al. (2005) was determined, using GC-FID and GC/MS, to possess the following composition:
α-pinene (1.1%)
myrcene (0.7%)
α-phellandrene (20.4%)
α-terpinene (1.3%)
p-cymene (3.6%)
1,8-cineole (10.3%)
δ-3-carene† (0.4%)
γ-terpinene (1.0%)
terpinolene (6.2%)
cis-α-bergamotene (0.2%)
(Z)-β-farnesene (0.4%)
α-humulene (0.2%)
γ-curcumene (0.7%)
ar-curcumene (2.9%)
zingiberene (6.9)
β-bisabolene (1.2%)
(Z)-γ-bisabolene (0.4%)
β-curcumene (0.5%)
β-sesquiphellandrene (5.5%)
α-turmerone (19.8%)
β-turmerone (7.4%)
ar-turmerone (1.1%)
†incorrect identification
Raina et al. (2005) used GC-FID and GC/MS to examine the rhizome oil of C. longa produced in the lab by hydrodistillation (0.8% oil yield from fresh rhizomes harvested from CIMAP field station in Pantnagar, Uttarakhand, India). The composition of this oil was determined to be as follows:
α-pinene (0.4%)
β-pinene (0.1%)
2-octanol (0.4%)
α-phellandrene (9.4%)
δ-3-carene (0.2%)
α-terpinene (0.1%)
p-cymene (1.2%)
limonene (0.2%)
1,8-cineole (1.9%)
γ-terpinene (0.4%)
terpinolene (1.2%)
linalool (0.1%)
camphor (0.1%)
myrtenal (0.1%)
cis-sabinol (0.3%)
neral (0.2%)
sabinyl acetate* (0.2%)
tetradecane (0.1%)
α-elemene (0.2%)
β-farnesene* (0.3%)
ar-curcumene (0.5%)
zingiberene (2.3%)
α-selinene (0.1%)
β-curcumene (0.4%)
β-sesquiphellandrene (1.8%)
(E)-nerolidol (0.2%)
ar-turmerol (0.1%)
caryophyllene oxide (0.4%)
epi-curzerenone (0.3%)
viridiflorol (1.6%)
trans-sesquisabinene hydrate (0.2%)
humulene epxoide II (0.6%)
10-epi-γ-eudesmol (0.2%)
T-cadinol (1.7%)
ar-turmerone (5.4%)
α-turmerone (44.1%)
germacrone (0.4%)
β-turmerone (18.5%)
geranyl hexanoate (0.6%)
(E)-α-atlantone (1.1%)
furanodienone (0.2%)
heptyl salicylate (0.7%)
(E)-cinnamyl cinnamate (0.1%)
*correct isomer not identified
In addition, octane, α-thujene, camphene, sabinene, (E)-β-ocimene, trans-p-menth-2-en-1-ol, p-cymen-8-ol and geranyl butyrate were found as trace constituents (< 0.05%) in this turmeric rhizome oil.
An oil produced by hydrodistillation in 2.2% yield from dried turmeric rhizomes was studied by Singh et al. (2005). Analysis using GC-FID and GC/MS revealed the oil possessed the following composition:
toluenea (0.1%)
myrcene (0.1%)
α-phellandrene (0.1%)
δ-3-carene (0.1%)
α-terpinene (0.1%)
p-cymene (0.2%)
limonene (0.2%)
1,8-cineole (0.3%)
terpinolene (2.3%)
p-cymen-8-ol (0.3%)
cumin alcoholb (0.1%)
β-caryophyllene (2.8%)
α-humulene (0.6%)
(E)-α-farnesene (0.2%)
ar-curcumene (3.8%)
γ-curcumene (0.1%)
zingiberene (2.6%)
β-bisabolene (0.8%)
β-curcumene (0.2%)
β-sesquiphellandrene (3.7%)
(E)-γ-bisabolene (0.1%)
(E)-nerolidol (0.5%)
caryophyllene oxide (0.7%)
zingiberenol (0.3%)
ar-turmerone (49.1%)
β-turmerone (2.4%)
α-turmerone (11.6%)
a600 ppm
balso known as p-cymen-7-ol
Using an efficient, economical and safe in vitro propagation procedure for turmeric, Ma and Gang (2006) examined the volatiles of the one-year-old rhizomes using GC/MS only. The constituents characterized (no quantitative data was given) were:
α-pinene; citronellene*; camphene; β-pinene; α-phellandrene; γ-3-carene; α-terpinene; o-cymene*; sylvestrene*; 1,8-cineole; α-terpinene; terpinolene; linalool; (Z)-cinerone*; trans-carveol*; 3,6,6-trimethyl-2-norpinanone*; 2-ethylidene-6-methyl-3,5-heptadiene*; dihydrocarvone isomer; γ-terpineol; cis-sabinol; trans-chrysanthenyl acetate*; bornyl acetate; piperitone oxide* isomer; ascaridole*; β-elemene; 7-epi-sesquithujene*; β-caryophyllene; cis-α-bergamotene; α-humulene; (Z)-β-farnesene; ar-curcumene; (E, E)-α-farnesene; zingiberene; β-bisabolene; β-sesquiphellandrene; (E)-8-bisabolene; cis-sesquisabinene hydrate; trans-sesquisabinene hydrate; turmerone isomer; g-curcumene; curlone; curcuphenol; α-bisabolene oxide*; corymbolone*; hexadecanoic acid; hexadecane-1,2-diol*; methyl linoleate; phytol; linoleic acid; oleic acid; linolenic acid; and octadecanoic acid.
The compounds listed with an asterisk were misidentified.
Tognolini et al. (2006) screened a number of commercial oils for their antiplatelet activity using guinea pig and rat plasma. Analysis of turmeric rhizome oil, which was one of the oils screened, using GC-FID and GC/MS revealed it possessed the following composition:
α-pinene (1.1%)
myrcene (0.7%)
α-phellandrene (20.4%)
δ-3-carene (0.4%)
α-terpinene (1.3%)
p-cymene (3.6%)
1,8-cineole (10.3%)
g-terpinene (1.0%)
terpinolene (6.2%)
trans-α-bergamotene (0.2%)
(Z)-β-farnesene (0.4%)
α-humulene (0.2%)
g-curcumene (0.7%)
ar-curcumene (2.9%)
zingiberene (6.9%)
β-bisabolene (1.2%)
(Z)-g-bisabolene (0.4%)
β-curcumene (0.5%)
β-sesquiphellandrene (5.5%)
ar-turmerol (0.9%)
α-turmerone (19.8%)
β-turmerone (7.4%)
ar-turmerone (1.1%)
Qin et al. (2007) examined the eight characteristic volatile constituents of C. longa grown in various warmer parts of China. The constituents were b-caryophyllene, ar-curcumene, zingiberene, β-bisabolene, β-sesquiphellandrene, ar-turmerone, α-turmerone and b-turmerone. The authors determined the optimum marker compounds for judging C. longa quality were ar-curcumene, ar-turmerone, α-turmerone and β-turmerone.
Asghari et al. (2009) obtained turmeric rhizomes from Chulalankara University, Thailand, and cultivated them in a greenhouse in Isfahan, Iran. Mature rhizomes were dried and ground to a fine powder and hydrodistilled to yield an oil. The oil, which was analyzed using GC-FID and GC/MS, was determined to contain the following major constituents:
α-phellandrene (2.2%)
α-terpinene (0.2%)
p-cymene (0.4%)
1,8-cineole (0.4%)
terpinolene (1.5%)
β-caryophyllene (0.6%)
ar-curcumene (0.8%)
zingiberene (1.5%)
β-bisabolene (0.4%)
β-sesquiphellandrene (1.3%)
ar-turmerone (68.9%)
α-turmerone (20.9%)
Fresh rhizomes of turmeric were obtained from Ilorin, Kwara State, Nigeria, pulverized and subjected to 3 hr hydrodistillation to produce an oil in 1.24% yield. This oil, which was analyzed using GC-FID and GC/MS by Usman et al. (2009), was determined to possess the following constituents:
α-thujene (6.7%)
α-pinene (2.8%)
β-pinene (2.4%)
myrcene (7.6%)
δ-2-carenea (4.0%)
β-phellandreneb (3.1%)
limonene (5.3%)
1,8-cineole (6.9%)
(Z)-β-ocimene (2.6%)
(E)-β-ocimene (9.8%)
isoartemisia ketone† (1.1%)
γ-terpinene† (2.6%)
borneol (3.3%)
terpineol* (2.1%)
α-terpineol (2.0%)
thymol† (6.4%)
zingiberene (5.2%)
β-bisabolene (13.9%)
β-sesquiphellandrene (5.2%)
turmerone* (3.5%)
*correct isomer not identified
†incorrect identification
ashould be δ-3-carene
bshould be α-phellandrene
Gardini et al. (2009) screened a few oils for their bactericidal and bacteriostatic effects against some pathogenic bacteria. One of the oils screened, produced (presumably) from turmeric grown in Cameroon, was analyzed by GC/MS only. The constituents characterized in this oil were:
α-phellandrene (4.0%)
p-cymene (0.6%)
1,8-cineole (2.8%)
terpinolene (0.4%)
sotolone† (0.4%)
(Z)-β-farnesene (0.5%)
β-cubebene† (2.0%)
zingiberene (3.5%)
β-bisabolene (0.3%)
γ-cadinene† (2.3%)
p-tert-octylphenol† (0.7%)
ar-turmerone (17.6%)
α-turmerone (43.1%)
curlone (17.5%)
caryophyllene oxide (0.5%)
†incorrect identification
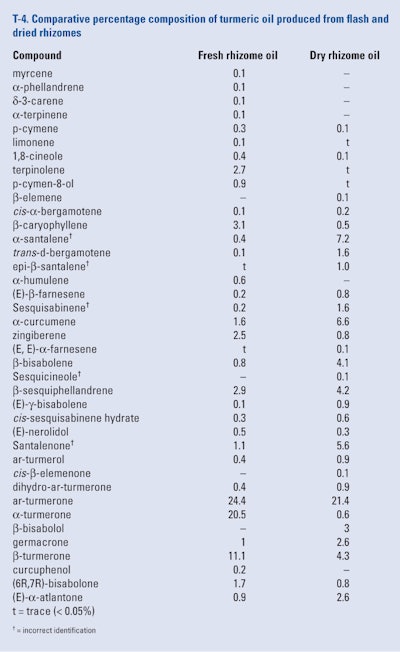
Singh et al. (2010) compared the chemical composition of oils produced from fresh and dried turmeric rhizomes. The rhizomes used were obtained from local farmers in the Gorakhpur area, U.P., India. A portion of the rhizomes were washed, air-dried and thinly grated (i.e., fresh rhizomes), while the dry rhizomes were washed, sun-dried and pulverized to a fine powder. Hydrodistillation of both the fresh and dried rhizomes for 5 hr yielded a fresh oil and a dry rhizome oil in yields of 1.4% and 2.9%, respectively. The two oils were analyzed using GC/MS only and the results of the analyses are shown in T-4.
Turmeric rhizomes of the ‘Roma’ cultivar were collected from the High Altitude Research Station Pottang, Koraput, Orissa, in the Eastern Ghats and grown in an experimental garden in Bhubaneswar, Orissa, India, by Singh et al. (2011). An oil produced from the freshly washed, cleaned and sliced fresh rhizomes by hydrodistillation for 10 hr was subjected to analysis by GC-FID and GC/MS. The main constituents of this oil were determined to be:
α-phellandrene (5.3%)
δ-3-carene (0.3%)
1,8-cineole (2.6%)
β-caryophyllene (0.8%)
β-farnesene* (0.6%)
ar-curcumene (3.5%)
β-bisabolene (0.6%)
β-sesquiphellandrene (1.7%)
ar-turmerone (49.1%)
β-turmeronea (16.8%)
*correct isomer not identified
aalso known as curlone
Dried turmeric rhizomes, which were imported into Taiwan from Sichuan Province, China, were ground and subjected to simultaneous steam-distillation and solvent extraction using the method of Likens and Nickerson (Tsai et al. 2011). The oil was analyzed using retention indices and GC/MS only. The constituents characterized in the oil were as follows:
α-pinene (0.1%)
β-pinene (0.2%)
limonene (0.1%)
1,8-cineole (2.9%)
p-cymene (0.8%)
2-heptanol† (0.1%)
linalool oxide*f (0.1%)
2-nonanol (< 0.1%)
camphor (0.1%)
linalool (0.5%)
β-elemene (0.9%)
terpinene-4-ol (0.2%)
β-caryophyllene (0.1%)
α-humulene (3.4%)
α-terpineol (2.9%)
α-selinene† (3.1%)
β-selinene† (10.2%)
δ-cadinene† (0.7%)
caryophyllene oxide (5.6%)
humulene oxide* (16.6%)
ar-turmerone (49.0%)
curcumol (1.5%)
dodecanoic acid (0.9%)
*correct isomer not identified
†questionable identification
An interesting review on the product quality of turmeric was reported by Li et al. (2011). An oil of turmeric root of Thai origin produced in 0.56% yield was screened for its lavicidal effect on Anopheles cracens (mosquito) by lntirach et al. (2012). Analysis of this oil using GC/MS only revealed it possessed the following major components:
p-cymene (0.9%)
1,8-cineole (0.9%)
β-caryophyllene (1.6%)
ar-curcumene (9.5%)
zingiberene (3.9%)
β-bisabolene (2.3%)
β-sesquiphellandrene (8.6%)
α-cedrene (0.8%)
ar-turmerone (30.2%)
turmerone (19.0%)
curlonea (13.3%)
aalso known as (6S)-2-methyl-6-[(1S)-4-methylene-2-cyclohexen-1-yl]-2-hepten-4-one, or bisabda-2,4(1S), 10-trien-9-one
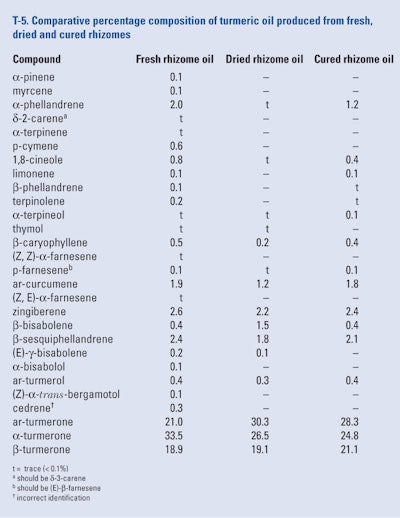
Fresh, mature turmeric rhizomes were obtained from a farmer near Mysore, Karnataka, India. They were cleaned, grated, blended with water and hydrodistilled for 8 hr. Similarly, dried rhizomes and cured rhizomes (i.e., fresh rhizomes cooked in water, shade-dried and polished) were subjected to a hydrodistillation process (Gounder and Lingamallu, 2012). The oil yields were 3.52% (fresh), 3.05% (dried) and 4.45% (cured). Each oil was separately analyzed using GC-FID and GC/MS and the results of the analyses can be seen in T-5.
An oil produced from the rhizomes of C. longa grown in Colombia was analyzed by Coy Barrera et al. (2013) using GC/MS and retention indices only. They found that the oil contained the following constituents:
turmerone (36.9%)
α-turmerone (18.9%)
β-turmerone (13.6%)
ar-curcumene (1.8%)
borneol (0.9%)
citral* (0.3%)
zingiberene (0.5%)
(Z,Z)-farnesol (0.3%)
zingiberenol* (0.2%)
β-caryophyllene (0.1%)
caryophyllene oxide (< 0.1%)
*correct isomer not identified
Finally, Lee et al. (2014) examined the volatile profile of C. longa using a combination of modern analytical techniques. Although the authors did not present any quantitative data, they did identify the following constituents in the oil: terpinolene, (Z)-β-farnesene, α-humulene, germacrene D, β-bisabolene, β-sesquiphellandrene α-patchoulene, cedr-9-ene, ar-turmerone α-turmerone and curlone.
References
B. Marongiu, S. Porcedda, A. Caredda, B. De Gioannis and A. Piras, Supercritical CO2 extraction of curcumin and essential oil from the rhizomes of Turmeric (Curcuma longa L.). J. Essent. Oil Bear. Plants, 5, 144–153 (2002).
N. K. Leela, A. Tava, P. M. Shafi, S. P. John and B. Chempakami, Chemical Composition of essential oils of turmeric (Curcuma longa L.) Acta Pharm., 52, 137–141 (2002).
M. E. M. Braga, P. F. Leal, J. E. Carvalho and M. A. A. Meireles, Comparison of yield, Composition and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem., 51, 6604–6611 (2003).
G. Singh, I. P. Kapoor, S. K. Pandey and O. P. Singh, Curcuma longa – chemical, antifungal and antibacterial investigation of rhizome oil. Indian Perfum., 47(2), 173–178 (2003).
G. Sacchetti, S. Maielti, M. Muzzoli, M. Scaglianti, S. Manfredini, M. Radice and R. Bruni, Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem., 91, 621–632 (2005).
V. K. Raina, S. K. Srivastava and K. V. Syamsundar, Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J. Essent. Oil Res., 17, 556–559 (2005).
G. Singh, S. Maurya, C. A. N. Catalan and M. P. de Lampasona, Chemical, antifungal, insecticidal and antioxidant studies on Curcuma longa essential oil and its oleoresin. Indian Perfum., 49, 441–451 (2005).
X–Q. Ma and D. R. Gang, Metabolic profiling of turmeric (Curcuma longa L.) plants derived from in vitro micropropagation and conventional greenhouse cultivation. J. Agric. Food Chem., 54, 9573–9583 (2006).
M. Tognolini, E. Barocelli, V. Ballabeni, R. Bruni, A. Bianchi, M. Chiavarini and M. Impicciatore, Comparative Screening of plant essential oils: phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci., 78, 1419–1432 (2006).
N-Y. Qin, F-Q Yang, Y-T. Wang and S-P. Li, Quantitative determination of eight components of rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using Pressurized liquid extraction and gas chromatography – mass spectrometry. J. Pharmaceut. Biomed. Anal., 43, 486–492 (2007).
B. Chempakam and V. A. Parthasarathy, Turmeric. In: Chemistry of Spices. Edits., V. A. Parthasarathy, B. Chempakam and T. J. Zachariah, pp. 97–123, CAB, Wallingford, UK (2008).
G. Asgari, A. Mostajeran and M. Shebli, Curcumoid and essential oil components of turmeric at different stage of growth cultivated in Iran. Res. Pharmaceut. Sci., 4(1), 55–61 (2009).
L. A. Usman, A. A. Hamid, O. C. George, O. M. Ameen, N. O. Muhammad, M. F. Zubair and A. Lawal, Chemical composition of rhizome essential oil of Curcuma longa L. growing in North Central Nigeria. World J. Chem., 4(2), 178–181 (2009).
F. Gardini, N. Belletti, M. Ndagijimana, M. E. Guerzoni, F. Tchoumbougnang, P. H. Amvam Zollo, C. Micci, R. Lanciotti and S. L. Sado Kamdem, Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria Monocytogenes, Salmonella enteritidis and Staphylococcus aureus. Afr. J. Microbiol. Res., 3, 264–271 (2009).