Myrica gale is a 1–2 m high deciduous flowering shrub with sweet, resinous, aromatic foliage that can be found typically growing in acidic peat bogs in northern and western Europe, particularly Scotland, Finland and parts of North America. Myrica gale grows well in less fertile soils because there is a symbiotic relationship between the plant and an endophytic nitrogen-fixing fungus (Simpson et al. 1996). In the 16th century, the leaves were commonly used in a mixture with so-called wild rosemary (Rhododendron tomentosum [Stokes]) H. Hamaja (syn. Ledum palustre L.) known as fruit as a flavoring for beer (Behre, 1983). Even though the leaves, catkins and fruit are aromatic, M. gale is grazed by sheep, goats, cattle and deer. The leaves have also been used as a traditional insect repellent (Simpson et al. 1996). An oil is occasionally produced in Canada and in northern European countries by steam distillation of the leaves of Myrica gale L. (syn. Gale palustris A. Mev.).
Von Schantz and Kapetanidis (1971) examined the composition of an oil of M. gale leaves collected in Finland. Using a combination of column chromatography and GC/MS analysis, the oil was found to possess the following composition:
α-pinene (17.8%)
camphene (0.7%)
β-pinene (1.7%)
myrcene + α-phellandrene (6.4%)
α-terpinene (1.0%)
limonene (10.0%)
1, 8-cineole (7.1%)
β-phellandrene (3.2%)
(E)-β-ocimene (1.1%)
α-terpinene (0.6%)
p-cymene (4.4%)
terpinolene (0.8%)
linalool (0.4%)
α-copaene (2.8%)
guaia-6,9-diene† (0.7%)
bornyl acetate (0.2%)
terpinen-4-ol (1.1%)
β-caryophyllene (2.6%)
α-elemene† (0.2%)
α-terpineol (2.1%)
longifolene (0.2%)
ε-cadinene† (0.9%)
α-terpinyl acetate (0.7%)
allo-aromadendrene (0.4%)
γ-muurolene (0.3%)
ε-muurolene† (0.2%)
α-muurolene (1.1%)
δ-cadinene + γ-cadinene (12.9%)
calamenene* (2.4%)
calacorene* (0.3%)
nerolidol* (5.9%)
α-muurolol (0.2%)
*correct isomer not identified
†identification requires corroboration
In addition, tricyclene (0.1%) was tentatively identified in this same oil.
An oil produced from the dried leaves of M. gale collected in Ontario, Canada was analyzed by GC-FID and retention times only (Halim and Collins, 1973). The compounds characterized in the oil were:
α-pinene (3.0%)
camphene (0.1%)
β-pinene (0.1%)
myrcene (16.2%)
α-terpinene (0.1%)
p-cymene (4.7%)
limonene (10.8%)
1, 8-cineole (2.8%)
(Z)-β-ocimene (0.9%)
(E)-β-ocimene (1.2%)
γ-terpinene (0.5%)
linalool (0.7%)
terpinen-4-ol (0.9%)
α-terpineol (2.1%)
α-copaene (0.5%)
β-caryophyllene (0.1%)
selina-4,11-diene (6.0%)
β-bisabolene (3.0%)
calamenene* (0.3%)
β-cadinene (2.5%)
(E)-nerolidol (1.0%)
caryophyllene oxide (1.8%)
selina-11-en-4-ol (14.6%)
α-bisabolol (5.0%)
*correct isomer not identified
Fresh leaves of M. gale were collected from the vicinity of Barry’s Bay, Ontario, Canada, dried and subjected to hydrodistillation to yield an oil that was analyzed by a combination of column chromatography, preparative GC and infrared spectroscopy (Lawrence and Weaver, 1974). The constituents characterized in this oil were as follows:
α-thujene (0.5%)
α-pinene (3.0%)
β-pinene (0.1%)
myrcene (16.2%)
α-phellandrene (5.1%)
α-terpinene (0.1%)
limonene (10.8%)
1, 8-cineole (2.8%)
(Z)-β-ocimene (0.9%)
(E)-β-ocimene (1.2%)
γ-terpinene (0.5%)
p-cymene (4.7%)
terpinolene (0.2%)
6-methyl-5-hepten-2-one (0.1%)
α-copaene (0.5%)
linalool (0.7%)
trans-α-bergamotene (1.3%)
terpinen-4-ol (0.9%)
selina-4,11-diene (6.0%)
carvotanacetone (0.2%)
α-terpineol (2.1%)
α-terpinyl acetate (0.2%)
(E)-β-farnesene (1.0%)
α-muurolene (0.2%)
α-selinene + β-selinene (0.1%)
piperitone (0.1%)
β-bisabolene (3.0%)
carvone (0.1%)
geranyl acetate (0.3%)
α-farnesene* (0.1%)
δ-cadinene + γ-cadinene (2.5%)
ar-curcumene (0.2%)
cubebene† (0.3%)
calamenene* (0.3%)
caryophyllene oxide (1.8%)
(E)-nerolidol (1.0%)
humulene epoxide II (0.8%)
α-bisabolol (5.0%)
selin-11-en-4α-ol (14.6%)
*correct isomer not identified
†incorrect identification
In addition, trace amounts (<0.1%) of camphene, amyl acetate, p-cymenene, α-cubebene, octyl acetate, benzaldehyde, β-caryophyllene, guaia-6,9-diene, δ-selinene and benzyl isovalarate were also identified in this oil.
An oil produced from M. gale leaves of the Netherlands origin was the subject of analysis by Tattje and Bos (1974). The compounds characterized in this oil were:
α-pinene (25.0%)
camphene (1.0%)
β-pinene (2.5%)
myrcene (1.3%)
α-terpinene (0.5%)
p-cymene (6.0%)
limonene (5.0%)
β-phellandrene (1.0%)
1,8-cineole (20.0%)
(Z)-β-ocimene (0.1%)
(E)-β-ocimene (0.5%)
γ-terpinene (0.5%)
linalool (0.5%)
terpinen-4-ol (2.2%)
α-terpineol (0.5%)
nerol (7.7%)
β-elemene (1.0%)
β-caryophyllene (0.5%)
α-humulene (3.5%)
nerolidol* (1.7%)
β-elemenone* (1.3%)
*correct isomer not identified
An oil produced from the leaves of M. gale collected in Spain by Velasco Negueruela et al. (1982) was analyzed by GC-FID and retention times only. The constituents characterized in this oil and confirmed by IR were:
α-pinene (13.3%)
camphene (1.0%)
β-pinene (0.3%)
myrcene (1.9%)
α-phellandrene (0.6%)
α-terpinene + p-cymene (4.0%)
limonene (12.3%)
1,8-cineole (20.0%)
γ-terpinene (2.4%)
terpinolene (0.6%)
linalool (0.5%)
borneol (0.5%)
terpinen-4-ol (2.9%)
α-terpineol (3.6%)
bornyl acetate (0.3%)
α-terpinyl acetate (0.2%)
β-caryophyllene (1.0%)
A trace amount of α-thujene (0.1%) was also found in this oil.
The constituents reported in an oil obtained from the leaves of M. gale by Carlton et al. (1992) were:
α-pinene (12.3%)
camphene (0.4%)
β-pinene (1.8%)
myrcene (1.4%)
α-terpinene (0.1%)
p-cymene (1.1%)
limonene (1.5%)
1,8-cineole (10.6%)
(E)-β-ocimene (0.2%)
γ-terpinene (0.4%)
linalool (0.4%)
terpinen-4-ol (0.1%)
α-terpineol (0.1%)
β-caryophyllene (0.2%)
α-humulene (0.1%)
selina-4,11-diene (0.1%)
germacrene D (11.6%)
β-cadinene (3.9%)
nerolidol* (4.0%)
isobarbatene ketone† (4.4%)
β-elemenone* (14.3%)
*correct isomer not identified
†incorrect identification
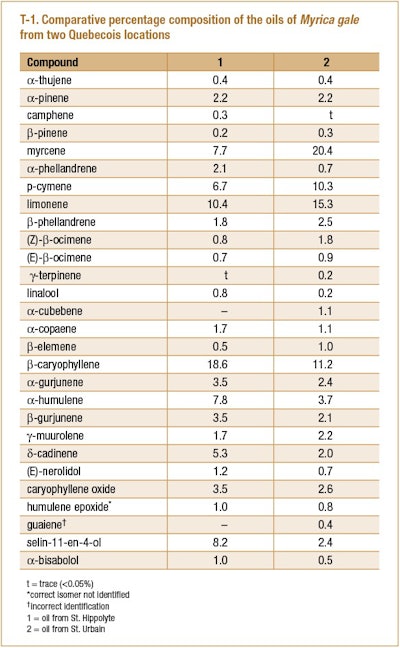
Bélanger et al. (1996) analyzed the lab-distilled oils of the leaves and stems of M. gale harvested from Canadian wild plants growing in the vicinity of St. Hippolyte, (northern Quebec) and St. Urbain (southern Quebec). Oils from each location were produced by hydrodistillation for 4 hr. A comparison between the compositions of the two oils is presented in T-1.
In a follow-up report, Bélanger et al. (1997) used a combination of GC-FID and GC/MS to examine the composition of a commercial oil of sweet gale (also known as wax myrtle). They found that the oil contained the following constituents:
1-hexene (0.2%)
2-propanone (0.2%)
1-buten-3-one (0.5%)
α-pinene (2.8%)
α-thujene (0.2%)
sabinene (0.2%)
β-pinene (0.3%)
myrcene (6.4%)
limonene (8.9%)
1,8-cineole (2.1%)
p-cymene (8.8%)
menthone (0.3%)
α-ylangene (0.3%)
isomenthone (0.1%)
α-copaene (1.5%)
α-gurjunene (0.2%)
linalool (1.5%)
linalyl acetate (0.2%)
β-caryophyllene + β-elemene (2.5%)
isocaryophyllene + terpinen-4-ol (0.6%)
methyl thymol (0.4%)
2-undecanol (0.2%)
β-guaiene* (0.1%)
p-menth-2-en-1-ol* (1.0%)
menthol (0.8%)
α-humulene (2.4%)
selina-4,11-diene + α-bulgarene† (1.7%)
α-muurolene (0.3%)
α-terpinyl acetate (0.5%)
eremophilene (3.0%)
α-muurolene (0.5%)
β-bisabolene (0.2%)
cis-carveol + 2,6-dimethyl-octa-1,5,7-trien-3-ol (0.2%)
δ-cadinene (1.1%)
isobisabolene† (0.1%)
ar-curcumene (1.3%)
p-mentha-1 (7), 8-dien-2-ol (0.2%)
nerol (0.1%)
calamenene* (2.2%)
p-cymen-8-ol (0.1%)
geraniol (0.6%)
calacorene* (0.2%)
isocaryophyllene oxide (1.1%)
caryophyllene oxide (9.3%)
humulene epoxide* (3.7%)
nerolidol* (1.5%)
cubenol (0.8%)
thymol (1.4%)
carvacrol (0.4%)
selin-11-en-4-ol (10.2%)
α-bisabolol (0.9%)
*correct isomer not identified
†incorrect identification
Trace amounts (<0.1%) of tricyclene, α-fenchene, camphene, α-phellandrene, β-phellandrene, (Z)-β-ocimene, γ-terpinene, (E)-β-ocimene, 2-ethyl-6-methylhepta-1,5-diene, p-cymenene, cis-limonene oxide, trans-limonene oxide, both furanoid forms of cis- and trans-linalool oxides, β-bourbonene, β-gurjunene, bornyl acetate an isomer of p-methyl-2-en-1-ol, trans-pinocarveol, p-allylanisole, cis-piperitol, cis-sabinol and α-muurolol were also characterized this oil.
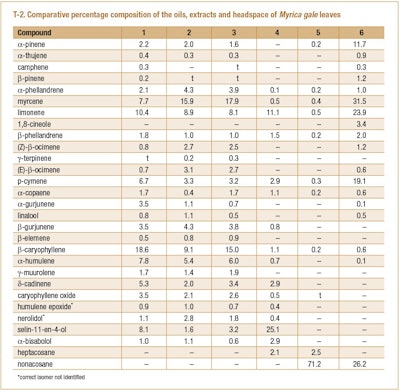
The same authors also compared the compositions of oils produced by steam-distillation both lab-scale and industrial-scale and hydrodistillation with a supercritical fluid CO2 extract, a microwave assisted hexane extract and the headspace of the leaves kept in an enclosed flask for 1 hr at 60°C.
The results of the analyses of the oils, extracts and leaf headspace are shown in T-2. It is interesting to note the quantitative difference between certain constituents in the oils produced by different processes.
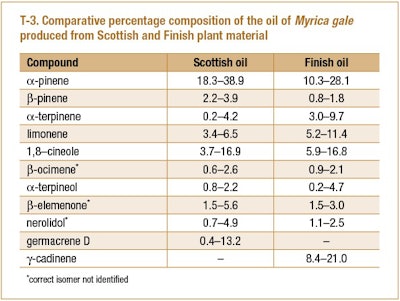
Svoboda et al. (1998) examined the effect of harvest data on the main components of sweet gale oil produced from the flowering tops of plants collected in Scotland and Finland. A summary of the range of data can be seen in T-3.
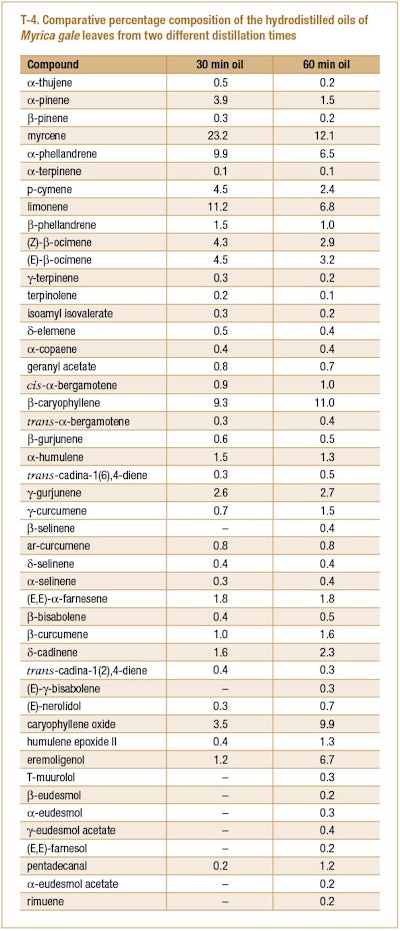
Silvestre et al. (2005) collected the leaves of M. gale from the shore of Surprise Lake, Shipsaw, Quebec, Canada and subjected the fresh leaves to hydrodistillation for 1 hr. During the distillation, an oil was collected after 3 min (oil yield 0.45%) and at the end of the 1 hr distillation (oil yield 0.10%). The two oils were separately analyzed using GC-FID and GC/MS. The results of this study can be seen in T-4.
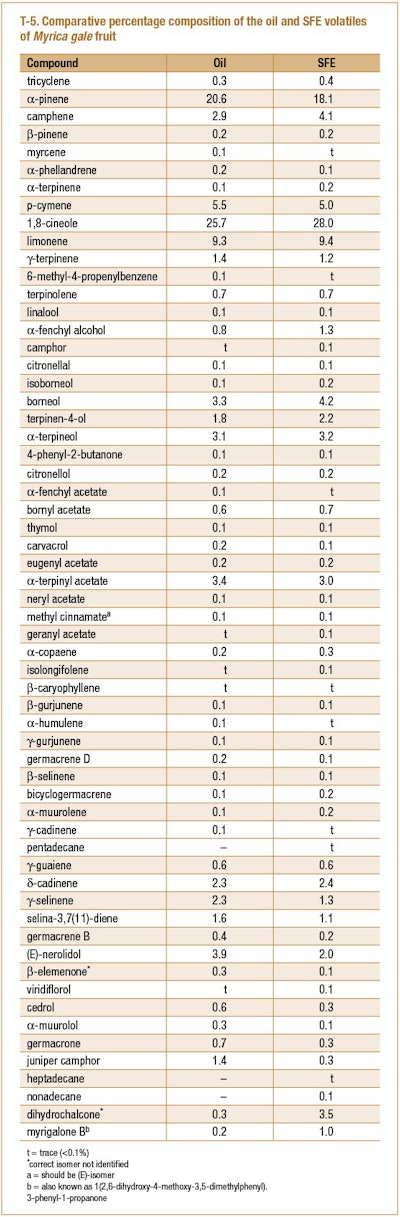
Myrica gale fruit collected from the island Saaremaa, Estonia and either distilled to produce an oil using a simultaneous distillation and extraction system or an extract (SFE) using supercritical fluid CO2 (Sokolova et al. 2005). Both the oil and extract were analyzed using GC-FID and GC/MS. A summary of their compositions can be seen in T-5.
Silvestre et al. (2006) analyzed another oil of M. gale that was produced by 2 hr hydrodistillation from leaves collected in Quebec, Canada. The authors used a combination of high pressure flash chromatography equipped with a silica gel column to produce four fractions of the oil. Analysis of the first fraction using GC-FID and GC/MS resulted in the characterization of the mono- and sesquiterpene hydrocarbons, while the other three fractions comprised oxygenated mono- and sesquiterpenes. Using the constituent characterizations from the analysis of each fraction, the authors were able to determine that the complete leaf oil contained the following:
α-thujene (0.3%)
α-pinene (2.2%)
camphene (0.1%)
sabinene (0.1%)
β-pinene (0.2%)
myrcene (11.3%)
α-phellandrene (7.1%)
α-terpinene (0.3%)
p-cymene (3.3%)
limonene (5.3%)
1,8-cineole (0.3%)
β-phellandrene (0.9%)
(Z)-β-ocimene (2.1%)
(E)-β-ocimene (2.2%)
α-terpinene (0.7%)
terpinolene (0.3%)
linalool (0.1%)
isoamyl isovalerate (0.2%)
terpinen-4-ol (0.1%)
octyl acetate (0.1%)
hexyl acetate (0.2%)
bornyl acetate (0.1%)
α-terpinyl acetate (0.6%)
α-copaene (0.2%)
geranyl acetate (0.2%)
β-elemene (0.4%)
cis-α-bergamotene (0.1%)
β-caryophyllene (8.4%)
α-humulene (2.9%)
germacrene D (0.9%)
γ-gurjunene (1.9%)
γ-curcumene (0.4%)
β-selinene (2.9%)
valencene (1.1%)
δ-selinene (0.9%)
α-selinene (2.4%)
(E,E)-α-farnesene (0.8%)
β-curcumene (0.3%)
δ-cadinene (1.1%)
trans-cadina-1(2), 4-diene (0.7%)
α-cadinene (0.2%)
selina-3, 7(11)-diene (0.6%)
germacrene B (1.2%)
(E)-nerolidol (1.1%)
caryophyllene oxide (1.1%)
humulene epoxide II (0.5%)
selin-11-en-4α-ol (11.5%)
germacrone (4.3%)
α-bisabolol (1.2%)
The commercial oil of M. gale, which was produced in Grondines, Quebec from Canadian plant material, was analyzed by Collin and Gagnon (2016) using GC-FID and GC/MS.
The composition of this oil was determined to be:
α-thujene (0.2%)
α-pinene (1.1%)
β-pinene (0.1%)
myrcene (7.9%)
α-phellandrene (3.8%)
α-terpinene (0.1%)
p-cymene (1.5%)
limonene (4.1%)
1,8-cineole (0.8%)
β-phellandrene (0.7%)
(Z)-β-ocimene (1.0%)
(E)-β-ocimene (1.1%)
γ-terpinene (0.3%)
terpinolene (0.1%)
linalool (0.2%)
isoamyl isovalerate (0.1%)
terpinen-4-ol (0.2%)
α-terpineol (0.1%)
α-terpinyl acetate (0.7%)
citronellyl acetate (0.2%)
α-copaene (0.2%)
geranyl acetate (0.2%)
β-elemene (0.3%)
benzyl isovalerate (0.3%)
cis-α-bergamotene (0.3%)
β-caryophyllene (5.6%)
γ-elemene (1.0%)
α-humulene (1.9%)
drima-7, 9(11)-diene (0.5%)
trans-cadina-1(6), 4-diene (0.5%)
β-chamigrene (0.8%)
γ-muurolene (0.7%)
γ-curcumene (0.8%)
β-selinene (1.1%)
ar-curcumene (1.1%)
α-selinene (1.2%)
zingiberene (0.8%)
(E,E)-α-farnesene (1.2%)
β-curcumene (0.9%)
δ-cadinene (2.0%)
trans-calamenene (0.2%)
selina-4(15), 7(11)-diene (1.2%)
selina-3, 7(11)-diene (2.6%)
germacrene B (7.5%)
cadina-1(10),6,8-triene (0.3%)
(E)-nerolidol (2.0%)
β-vetivenene (0.5%)
caryophyllene oxide (0.6%)
β-elemenone* (0.3%)
humulene epoxide II (0.3%)
eremoligenol (0.2%)
l-epi-cubenol (0.3%)
selin-11-en-4α-ol (2.3%)
α-bisabolol (2.2%)
(E,E)-germacrone (13.5%)
juniper camphor (0.1%)
guaiol acetate (0.4%)
pentadecanal (<0.1%)
α-eudesmol acetate (0.4%)
*correct isomer not identified
The authors also determined the hydrosol of M. gale stored over 24 months ranged in composition as follows:
3-hydroxy-2-butanone (0.1–1.5%)
(E)-3-hexenol (0.2–0.8%)
(Z)-3-hexenol (9.0–37.6%)
(E)-2-hexenol (1.1–2.3%)
hexanol (1.6–3.7%)
hexanoic acid (2.0–3.3%)
(Z)-3-hexenoic acid (<0.1%)
(E)-3-hexenoic acid (2.5–4.0%)
1,8-cineole (2.0–10.0%)
benzyl alcohol (3.0–4.4%)
(E)-2-hexenoic acid (0.8–3.7%)
γ-hexalactone (0.7–0.9%)
trans-linalool oxidef (0.2–0.5%)
octanol (0–0.9%)
fenchone (<0.1–0.3%)
heptanoic acid (<0.1%)
cis-linalool oxidef (0.2–0.3%)
linalool (0.3–5.9%)
α-thujone (0.2–0.4%)
maltol† (t–0.2%)
2-phenethyl alcohol (0.3–0.6%)
ipsdienol† (t–0.7%)
2,6-dimethyl-1,5,7-octadien-3-ol (0.9–2.2%)
isoborneol (0.6–1.0%)
p-menth-5-en-2-one* (0–0.2%)
borneol (0.3–1.1%)
p-menth-5-en-2-one* (0–0.4%)
benzoic acid (0–2.3%)
terpinen-4-ol (3.9–10.5%)
octanoic acid (0.1–0.8%)
cryptone (t–0.5%)
p-cymen-8-ol (0.5–1.7%)
methyl salicylate (0–0.7%)
α-terpineol (3.8–10.2%)
trans-sabinol (1.6–4.1%)
trans-piperitola (0–0.6%)
trans-carveol (0.1–0.6%)
nerol (0.1–0.6%)
citronellol (0–1.7%)
2,6-dimethyl-7-octene-2,6-diol (0–1.3%)
cis-sabinol (0.2–1.9%)
carvotanacetone (0.3–0.8%)
piperitone (0.3–0.8%)
geraniol (0.2–0.7%)
thymol (t–0.2%)
carvacrol (t–0.4%)
p-vinylguaiacol (0.1–0.4%)
p-menthane-1,8-diol (0.2–0.6%)
eugenol (0.1–0.2%)
benzylacetone† (0–0.2%)
hydroxycitronellol† (0.1–0.3%)
benzyl isovalerate (0–0.3%)
(E)-nerolidol (0–0.2%)
eremoligenol (0.1–1.3%)
γ-eudesmol (t–0.2%)
selin-11-en-4α-ol (0–2.3%)
(E,E)-germacrone (0.2–3.5%)
α-bisabolol (0–0.8%)
rosifoliol (0–0.6%)
t = trace (<0.1%)
f = furanoid form
a = tentative identification
†identity requires corroboration