A survey of the literature reveals that Jacobsen et al. (1987) determined that α-copaene, which was found as a constituent in ylang ylang oil, existed exclusively in the levorotatory form.
Sacchetti et al. (2005) obtained a number of commercial essential oils in Italy that were screened for their antioxidant, anti-radical and antimicrobial activities. Among the oils screened was cananga oil. Its composition was determined by GC-FID and GC/MS to be as follows:
α-pinene (0.4%)
sabinene (0.6%)
myrcene (0.1%)
p-cresyl methyl ether (0.8%)
p-cymene (0.1%)
limonene (0.1%)
1,8-cineole (0.1%)
benzyl alcohol (1.9%)
δ-terpinene (0.2%)
methyl benzoate (1.5%)
linalool (24.5%)
dihydrolinaloola (0.5%)
benzyl acetate (9.8%)
terpinen-4-ol (0.2%)
methyl salicylate (2.8%)
δ-terpineola (0.4%)
α-gurjunene (4.6%)
β-caryophyllene (0.4%)
thujopsene† (0.9%)
cuparene† (0.1%)
myristicin† (0.2%)
cedrol† (0.9%)
benzyl salicylate (12.9%)
benzyl benzoate (33.6%)
adoes not occur naturally
balso known as p-methyl anisole
†incorrect identification
It should be noted that the occurrence of dihydrolinalool in this oil indicates that the commercial oil was adulterated with synthetic linalool.
Kaiser (2006) reported that cananga flowers are grown for ceremonial purposes and cananga oil production by special communities in the villages of Purwodadi and Serengat near Malang (Eastern Java). The trees are grown using a mixed culture system where they are integrated with fruit trees and cocoa and coffee shrubs. The headspace analysis of the living flowers of C. odorata flowers obtained from Lakekamu Basin, Papua New Guinea was determined by Kaiser (2006).The constituents characterized in this headspace were as follows:
methyl 2-methylbutyrate (0.1%)
isobutyl acetate (0.1%)
α-pinene (0.2%)
butyl acetate (0.2%)
β-pinene (0.3%)
sabinene (0.4%)
isoamyl acetate (0.1%)
myrcene (0.6%)
limonene (0.2%)
isoprenyl acetate (0.4%)
isoprenol (0.1%)
(Z)-β-ocimene (1.1%)
(E)-β-ocimene (2.2%)
prenyl acetate (1.7%)
(E)-4,8-dimethylnona-1,3,7-triene (0.1%)
(Z)-3-hexenyl acetate (0.2%)
prenol (0.2%)
hexanol (0.1%)
nonanal (0.1%)
p-methyl anisole (4.2%)
α-cubebene (0.1%)
α-copaene (1.0%)
(E)+(Z)-isovaleraldehyde oxime (0.1%)
benzaldehyde (0.1%)
cis-sabinene hydrate (0.1%)
linalool (24.0%)
β-copaene (0.1%)
β-caryophyllene (7.0%)
methyl benzoate (1.2%)
α-humulene (1.0%)
methyl chavicol (0.2%)
germacrene D (21.0%)
benzyl acetate (6.8%)
(Z, E)-α-farnesene (0.1%)
(E, E)-α-farnesene (12.2%)
geranyl acetate (0.4%)
(E)-anethole (0.1%)
geraniol (0.3%)
benzyl alcohol (0.8%)
jasmone* (0.2%)
cubebol (0.1%)
creosol (0.1%)
caryophyllene oxide (0.1%)
methyl eugenol (0.1%)
germacrene D-4-ol (0.1%)
(E)-nerolidol (0.1%)
prenyl benzoate (0.1%)
(E)-cinnamyl acetate (0.1%)
1-nitro-2-phenylethane (0.7%)
(Z)-3-hexenyl benzoate (0.1%)
eugenol (0.1%)
(Z,E)-farnesal (0.1%)
elemicin (0.1%)
(E,E)-farnesal (0.4%)
(E,E)-farnesal acetate (0.1%)
3-phenylacetaldehyde oxime (0.2%)
methyl trans-(Z)-jasmonate (0.2%)
isoeugenol* (0.1%)
(E,E)-farnesol (0.3%)
benzyl benzoate (0.2%)
*correct isomer not identified
In addition, trace amounts (<0.05%) of α-thujene, 3-methyl-1-nitrobutene, (Z)-3-hexenol, (E)-2-hexenol, 1-octen-3-ol and methyl cis-(Z)-jasmonate were characterized in this ylang ylang flower headspace.
Furthermore Kaisar noted that this unique cananga possessed a lower level p-methyl anisole than found in other cananga flower selections. In addition, comparatively high levels of jasmone and methyl cis-(Z)-jasmonate were also found. He remarked that although this was the first time that these jasmonoid compounds had been found as constituents, their presence in the flower fragrance gave it an appealing softness.
As part of a screening program for the antioxidant activity of commercially available essential oils, Wei and Shibamoto (2007) determined that the major constituents found in a sample of ylang ylang oil were:
p-methyl anisole (1.6%)
linalyl acetate (8.3%)
β-caryophyllene (11.1%)
α-humulene (3.6%)
germacrene D (19.1%)
α-farnesene* (12.4%)
(E,E)-α-farnesene (12.6%)
nerol (1.5%)
cinnamyl acetate (4.8%)
α-cadinol (1.0%)
(E,E)-farnesyl acetate (1.7%)
(E,E)-farnesol (1.0%)
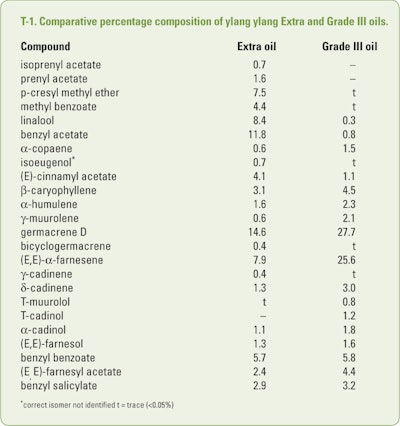
Williams (2008) reported the results of the analyses of ylang ylang oil III and Extra as can be seen in T-1. In addition, trace (<0.05%) amounts of butyl acetate, isoamyl acetate, 2-methylbutyl acetate, α-pinene, β-pinene, (Z)-3-hexenyl acetate, hexyl acetate, p-cresyl acetate, ethyl benzoate, methyl salicylate, α-terpineol, 2-phenethyl acetate, geraniol and α-ylangene were also characterized in the Extra oil. Only, α-cubebene was found as a trace constituent of Grade III oil, while β-ylangene was found as a trace constituent of both Extra and Grade III oils.
Hellivan (2008) reviewed the production, perfumery uses and commented on the composition of various grades of ylang ylang oil. Although it is well known, Hellivan reported that the grades of ylang ylang oil are as follows:
1. Ylang ylang Extra superior: oil produced after the initial 30 mins of distillation (SG: >0.965).
2. Ylang ylang Extra: oil produced after the next 60 mins of distillation (SG: 0.955–0.965).
3. Ylang ylang 1st (or 1): oil obtained after the third hour of distillation (SG: 0.945–0.955).
4. Ylang ylang 2nd (or 2): oil obtained after the fourth hour of distillation (SG: 0.932–0.940).
5. Ylang ylang 3rd (or 3): oil obtained after the sixth hour of distillation (SG: 0.905–0.910).
The constituents reported in ylang ylang oils were: prenyl acetate, p-cresol methyl ether, methyl benzoate, linalool, linalool, benzyl alcohol, geraniol, geranyl acetate, β-caryophyllene, (E)-cinnamyl acetate, α-humulene, germacrene D, (E,E)-α-farnesene, γ-cadinene, (E,E)-farnesol, benzyl benzoate, (E,E)-farnesyl acetate and benzyl salicylate. Unfortunately, the author did not report any quantitative data. Finally, the production level of ylang ylang oil in 2008 was reported as being Anjouan: 45–50 metric tonnes, Grande Comore (Comore islands): <2 mt., Moheli (Comore islands): 3 mt., Mayotte (France): 3–4mt., Nosi Bé (Madagascar): 17–20 mt and Ambanja (Madagascar): 3.5 mt.
Benini et al. (2010) reviewed the production of ylang ylang oil from the Indian ocean islands. They also reported the AFNOR (2005) data for the various quality oils, the data of which can be seen summarized in T-2.
Ranade (2011) reported that the main constituents of ylang ylang oil Extra were as follows:
α-pinene (0.3%)
linalool (10.3%)
linalyl acetate (0.2%)
β-caryophyllene (9.9%)
α-terpineol (0.1%)
farnesene* (18.0%)
p-methyl anisole (8.4%)
benzyl acetate (12.6%)
methyl anthranilate (0.1%)
geranyl acetate (4.0%)
cadinenes* (10.9%)
benzyl alcohol (0.5%)
geraniol (0.2%)
methyl salicylate (0.1%)
methyl benzoate (4.0%)
farnesols* (0.9%)
safrole (0.3%)
isoeugenol* (0.2%)
eugenol (0.2%)
p-cresol (0.1%)
benzyl benzoate (4.3%)
benzyl salicylate (1.9%)
*collect isomer not identified
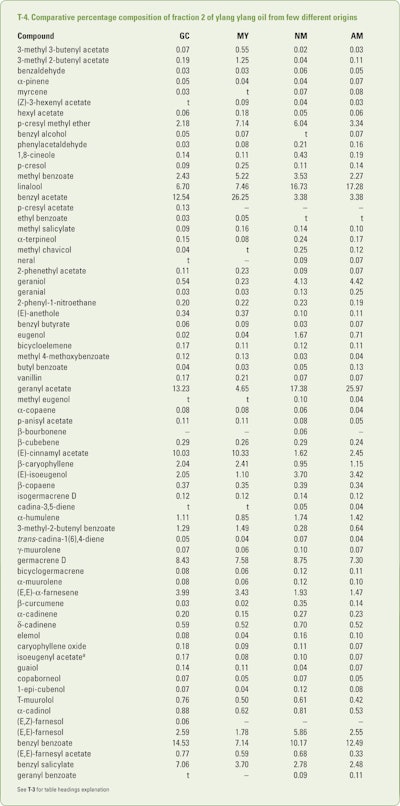
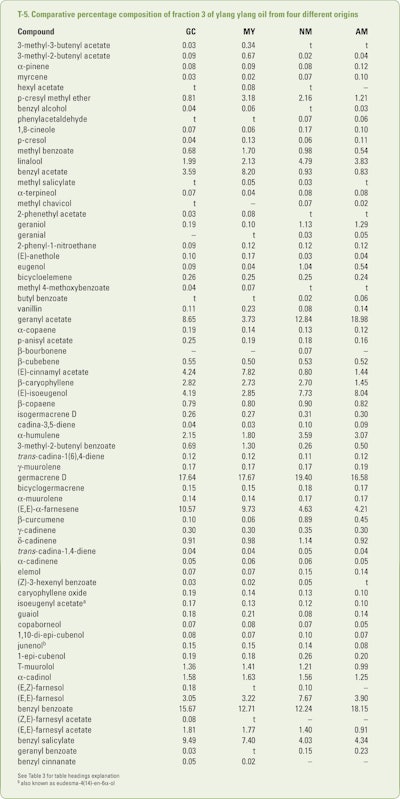
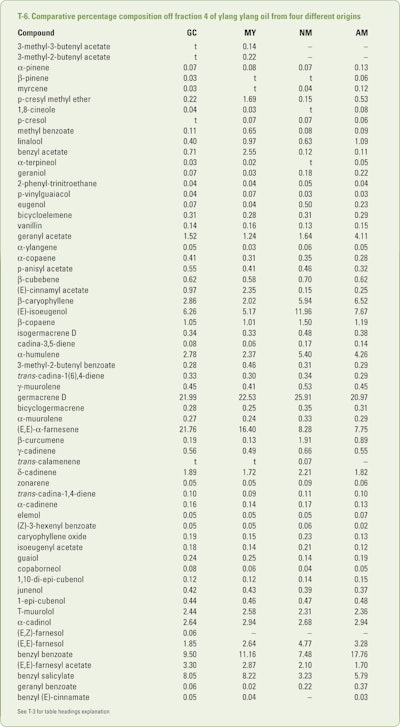
Benini et al. (2012a) collected (between 7–8 am) mature ylang ylang flowers in July, August and September from trees growing on Grand Comore, Mayotte, Nossi Bé and Ambanja. After subjecting separate hydrodistillations for each flower source, the resultant oils were collected in four separate fractions. These fractions were analyzed using GC-FID and GC/MS. The combined results obtained results obtained from the numerous analyses are shown in T-3 through T-6.
The data presentation in T-3 and T-4 are presented in two decimal places and have been included in the tables if a single constituent was found in an amount of 0.05% or greater. This was done because of the variations observed between the various fractions.
In addition, trace amounts (<.05%) of 3-methyl-3-butenol, 3-methyl-butenol, hexanol, 3-hexanol, (E)-2-hexanol, hexanol, isoamyl acetate, 2-methylbutyl acetate, heptanal, 6-methyl-5-hepten-2-one, α-thujene, m-cresol, cis-linalool oxide and trans-linalool oxide (furanoid forms), 2-methoxyphenol, terpinolene, phenylacetonitrile, veratrole, trans-linalool oxide (pyranoid form), terpinen-4-ol, 2-methoxy-4-methylphenol, nerol, 4(2-propenyllphenol, linalyl acetate, trans-linalool oxide acetate, (E)-cinnamyl alcohol, p-vinylguaiacol, nonyl acetate, methyl 2-methoxy-benzoate, 2-indanol, a 4-alkyl phenylacetate, neryl acetate, α-ylangene, isodauca-6,5-diene, cyperene, selina-4(15),5-diene, (E,Z)-farnesol, eicosane, tricosane and tetracosane were identified in one or more of the 12 oils. As expected the early fractions were richer in the more volatile constituents while the later fractions were richer in the high boiling constituents just as can be seen in T-6.
The compositions of the oils produced by hydrodistillations for hrs from fresh mature. ylang ylang flowers collected from five plantations (Adawa, Ifoundihé, Selea, Moubadjou and Mitsamiouli) in Grande Comore, four plantations (Bouyouni, Mavingoni, Vahibé and Dzoumogné) in Mayotte and two plantations (Tsaratarié and Amboimena) in Madagascar were examined by Benini et al. (2004) using GC-FID and GC/MS. The author’s determined that variations in the composition within plantations and between islands however there was not a genetic differentiation that could support the variation.
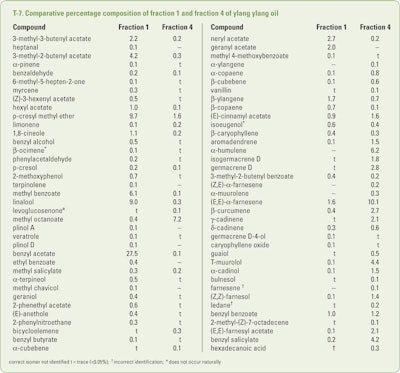
Brokl et al.(2013) collected mature ylang ylang flowers from seven different trees growing in the vicinity of Mavigoni (Mayotte). The pooled flowers were hydrodistilled for 8hr and four separate fractions: fraction 1 (after 25min), fraction 2 (after 1hr), fraction 3 (after 3hr) and fraction 4 (after 8hr.). Fractions 1 and 4 were subjected to GC × GC–TOFMS (Time of Flight Mass Spectrometry) and the findings of this study can be seen in T-7.
In addition. trace amounts (<0.05%) of decane, α-phellandrene, the furanoid form of cis-linalool oxide, phenylacetonitrile, 1-phenyl-2-propenol, 2-methoxy-4-methylphenol, dodecane, 1-methoxy-4-propylbenzene, 1-phenylallyl acetate*, geranial, diethyl 1,5-pentanedioate*, 1(H)-indole, vinyl butyrate*, (E)-cinnamyl alcohol, p-vinylguiacol, diethyl (2E)-3-methyl-2-pentanedioate*, 2,5-dimethyl-3-methylene-1,5-heptadiene*, methyl 2-methoxybenzoate, 5-indanol*, methyl 2-aminobenzoate*, 3-phenylpropyl acetate*, butyl benzoate, β-bourbonene, tetradecane, 2-methoxy-4-(l-propenyl)phenol, p-anisyl acetate*, 3-methyl-3-butenyl benzoate, α-ionene*, pentadecane, guaicyl acetone*, zonarene, benzyl 4-methylvalerate, elemol, (Z)-3-hexenyl benzoate, hexadecane, an isomer of isoeugenol acetate, copaborneol*, cetene*, (E,E)-farnesal, octadecane, octadecanal, eicosane, heneicosane, docosane, tricosane, tetracosane, pentacosane, hexacosane and heptacosane were characterized as constituents of both fractions, however, the constituents with an asterisk requires collaboration. Trace amounts of 3-hexenol, sabinene, the furanoid form of trans-linalool oxide, methyl isovalerate, nerol, 4-methoxy benzaldehyde, eugenol, octacosane and nonacosane were found as constituents of fraction 1. The trace amounts found only in fraction 4 were globulol, (Z)-α-bisabolene oxide, geranyl benzoate and benzyl cinnamate.
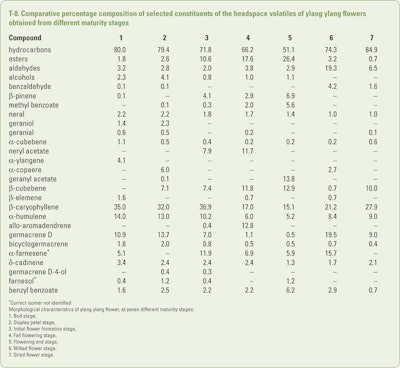
Qin et al. (2014) examined the change in chemical composition of the oils produced from C. odorata flowers from their bud stage through the full flowering stage to the wilted flower stage and the dried flower stage using headspace SPME-GC/MS. Unfortunately, the analyses did not make sense as the normally encountered major constituents of the early ylang ylang oil fractions, such as p-cresyl methyl ether, linaool and benzyl acetate, were either not identified or were found in miniscule amounts. Also, the authors reported that they characterized numerous constituents that were obviously incorrect computer-assisted identifications. This reviewer questions not only the identifications but also the repeatability of the analytical technique used. Nevertheless, a summary of a selected set of results can be seen in T-8. These results are presented so that in the future if such a study is repeated a comparison with the study of Qin et al. can be made.