The botanical origin of vetiver oil is Chrysopogon zizanoides Roberty [syn. Vetiveria zizanoides (L.) Nash] a member of the Poaceae (Graminae) family. Vetiver is endemic to India but was transported around the world more than a century ago. The original primary use for vetiver was in the treatment of soil erosion (Maffei, 2002). Although still used to treat soil erosion because of its prolific root system, it is grown in Haiti, Indonesia, China, India, Brazil, Dominican Republic, India, Vietnam, EI Salvador, Reunion, Madagascar, etc. for the production of an essential oil. It is estimated that the world production of vetiver oil is in excess of 250 metric tonnes. There are two main chemotypes of vetiver oil. The one produced in India (known a khus oil) originates from a fertile form and the one grown outside of India which is a non-seeding, sterile form. Khus oil has been used for decades in Indian fragrances (Parekh and Singh, 2009), while the rest of the world mainly uses oil produced from the sterile form.
Gupta et al. (2015) examined 40 accessions of vetiver from around the world to determine the genetic diversity and stability of Indian vetiver. They found that four genotypes from Razaganj, Ghoghraghat and Moradabad in Ultar Pradesh and Muzafferpur in Bihar were the most stable genotypes.
Hossé et al. (1995) used retention times (not indices) on both polar and non-polar capillary columns to determine that vetiver oil contained the following sesquiterpenes: prezizaene, khusimene, zizaene, khusimone, δ-muurolene, δ 2-cadinene, d-cadinene, (E)-nerolidol, β-vetivenene, T-cadinol, α-cadinol, khusinol, khusimol, zizanoic acid, β-vetivone and α-vetivone, Although the author’s presented some quantitative data, it is not included because it didn’t make much sense.
Chowdhury et al. (2002) used GC-FID and GC/MS to examine an oil produced by hydro distillation from the roots of vetiver collected from near Kanpur and Unnao (Uttarpradesh, India). The results of this analysis were poorly presented and contained numerous misidentifications, consequently the citation was only included for completeness of a literature review.
Adams et al. (2004) subjected the ‘Sunshine’ cultivar of vetiver to meristem tissue culture with the objective to produce plants which were free from fungi and bacteria grown from a rooted plantlet for five months in sterilized soil. In addition three plantlets taken directly from the original vetiver cultivar were grown in non-sterilized potting soil outside under ambient conditions in Waco, Texas. After the end of the summer season (mid-October) the roots from the sterile plants and the non-sterile plants were hydrodistilled to yield oils in 0.02% yield (sterile) and 0.29–0.40% yield (non-sterile) One oil from sterile roots (0.02%) and three oils from non-sterile roots (0.29–0.40%). Analysis of the four oils using GC/MS only revealed that the oils from the non-sterile roots possessed a typical vetiver oil chemical profile, while the oil from the sterile roots contained large amounts of alkanes and lacked the normally found fungal metabolites.
Although the authors did not characterize the complete chemical profile oil the oils they did show the differences were very pronounced. The non-sterile roots oils were determined to contain:
longicyclene (0–0.1%)
α-duprezianene (0–0.1%)
β-elemene (0–0.1%)
β-funebrene (0–0.2%)
guaia–6, 9–diene (0–0.1%)
(E)–isoeugenol (1.9–2.1%)
prezizaene (0.1%)
khusimene (0.6–1.3%)
α-amorphene (1.1–2.5%)
β-vetispirene (1.1–2.3%)
γ-amorphene (0.9–2.0%)
δ-vetivenene (0.2–0.5%)
elemol (0.4–0.5%)
β-vetivenene (2.0–3.7%)
α-cadinol (2.0–4.6%)
eudesm-7(11)-en-4-ol (0-1-0.8%)
nootkatol (0.8–1.8%)
vetiselinol (2.4–3.0%)
khusimol (13.1–24.6%)
(E)-isovalencenol (8.9–11.8%)
14-hydroxy-γ-cadinene (1.6–2.4%)
β-vetivone (2.1–2.7%)
α-vetivone (1.5–2.0%)
In contrast, the oil from the sterile roots was found to contain the following constituents:
o-guaiacol (0.2%)
decanal (<0.1%)
r-vinylguaiacol (1.0%)
(Z)-isoeugenol (<0.1%)
(E)-isoeugenol (6.4%)
khusimene (0.4%)
pentadecane (0.5%)
hexadecane (1.6%)
α-cadinol (0.2%)
epi-zizanone (2.4%)
tetradecanol (0.4%)
nootkatol (1.3%)
vetiselinenol (1.4%)
khusimol (11.5%)
(E)-isovalencenol (4.2%)
octadecane (0.8%)
14-hydroxy-γ-cadinene (0.8%)
β-vetivone (1.1%)
α-vetivone (0.9%)
nonadecane (0.5%)
hexadecanoic acid (1.7%)
eicosane (0.2%)
manool (0.2%)
heneicosane (0.6%)
docosane (2.4%)
tricosane (4.3%)
tetracosane (5.2%)
pentacosane (4.9%)
hexacosane (3.8%)
heptacosane (2.4%)
octacosane (1.4%)
nonacosane (0.7%)
Talansier etal. (2004) examined the kinetics of supercritical fluid CO2 extraction (SFE) of vetiver roots. They found that the optimum extraction pressure was 200 bar and 40°C with the inclusion of ethanol (10%) as a co-solvent resulting in an increase in total vetiver oil yield versus an extraction without the use of a co-solvent. The authors also characterized khusinol, isovalencenol, zizanoic acid and α- and β-vetivone as constituents of the SFE.
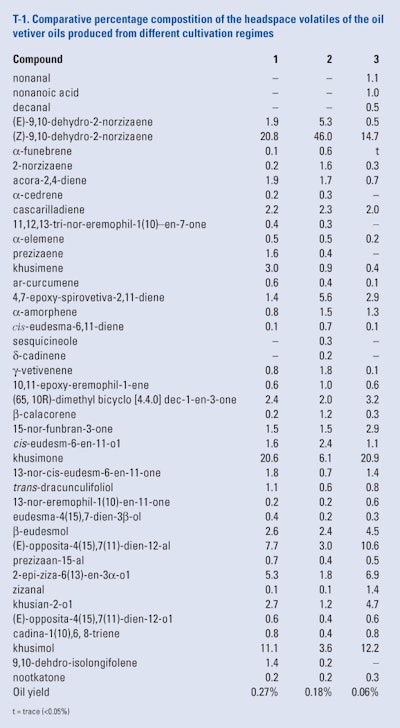
The roots of the ‘Mae Hae’ cultivar of vetiver (a common cultivar in Thailand) were planted in three different cultivation regimes. One in normal soil, one in normal soil that was injected with an arbuscular microrrhizal fungi, and one in a mixture of rice husks and compost in plastic baskets that were lowered into a hydroponic container. Five months later the roots from each cultivation regime were harvested, cleared, washed and dried and comminuted. Each root sample was subjected to a Likens-Nickerson SDE simultaneous distillation and extraction using methylene chloride as the solvent. Each oil was subjected to headspace analysis using SPME-GC/MS by Pripdeevech et al. (2006). The results of this study can be seen in T-1. Examination of these results reveals that the cultivation regimens affected the oil compositions particularly the quantities of the low molecular weight volatiles such as the 2-norzizaene derivatives.
Also, the hydroponically grown roots yielded an oil whose composition was similar to that grown in normal soil even though its oil yield was less than 25% of the oil yield of roots grown in normal soil.
Massardo et al. (2006) determined that the endophytic bacteria found associated with the roots of vetiver cultivated in Thailand were Pseudomonas, Kluyvera, Enterbacter, Serratia and Arthrobacter strain species. The authors categorically determined that the bacteria that were living inside the vetiver roots were capable of sesquiterpenoid biotransformations.
A commercial sample of vetiver oil that was purchased from a producer in Reunion was analyzed using GC-FID and GC/MS by Champagnat et al. (2007). The main constituents characterized in this oil were:
13-nor-eudesm-5-en-11-one (0.3%)
khusimonea (0.9%)
α-cadinol (4.0%)
cyclocopacamphan-12-o1 (4.0%)
2-epi-ziza-6(13)-en-12-al (1.0%)
trans-eudesma-4(15),7-dien-12-ol (3.0%)
khusimolb (13.3%)
preziza–7(15)-en-12-o1 (0.7%)
vetiselinenol (7.8%)
nootkatone (0.9%)
(Z)-isovalencenal (0.9%)
β-vetivone (4.2%)
(E)-isovalencenal (1.4%)
α-vetivone (6.4%)
aalso known as 12-nor-ziza-6(13)-en-2-one
balso known as ziza-6(13)-en-12-o1
It is well known that vetiver oil is one if not the most complex essential oils produced. As a result, Williams (2008) reported that only 35% of an oil of Haitian origin could be characterized. The constituents reported were:
α-amorphene (1.6%)
β-vetispirene (1.2%)
β-vetivenene (1.9%)
khusimol (12.8%)
isovalencenol (10.7%)
β-vetivone (3.6%)
α-vetivone (4.1%)
In addition, trace amounts (<0.05%) of prezizaene, zizaene and δ-vetivenene were also found in this oil.
Prata et al. (2008) extracted zizanoic acid from vetiver oil using IM. NaOH and methylene chloride. The sodium zizanoate was then released using HCl at PH 3.0 to deposit the acids into the organic layer which in turn were washed with water, dried over anhydrous sodium sulphate after which the solvent was removed under vacuum. The zizanoic acid, which was purified using silica gel flash chromatography, was reduced to khusimol using LiAlH4 in dry tetrahydrofuran. In addition the authors determined the quantitative amounts of some selected constituents such as:
preziza-7(15)-ene (1.1%)
khusimene (1.4%)
α-amorphene (2.3%)
cis-eudesma-6,11-diene (2.7%)
β-vetispirene (3.3%)
δ-vetivenene (2.3%)
khusimone (2.7%)
2-epi-ziza-6(13)-en-3α-o1 (2.5%)
khusian-2-o1 (1.6%)
trans-eudesma-4(15),7-dien-12-o1 (2.2%)
khusimol (9.3%)
α-vetivone (3.6%)
Chautian et al. (2008) registered ‘CIM-Vriddhi’ a high oil yielding cultivar of vetiver. This is a very late flowering cultivar that according to the author’s the oil content, was 1.69% with a potential oil production level of 30.57 kg/ha.
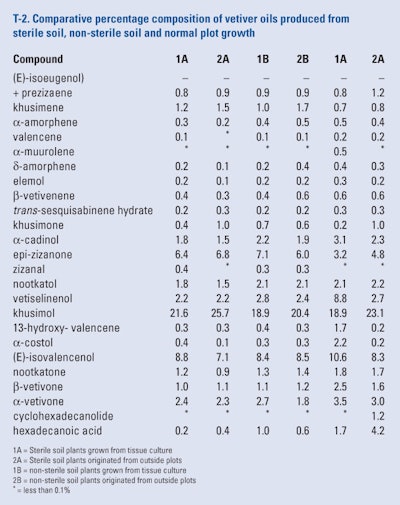
Adams et al. (2008) reported that two vetiver cultivars (‘Karnataka’ and ‘Malaysia’) were subjected to meristem tissue culture to yield clean (bacteria and fungi-free) plants that in turn were pot grown in sterile and unsterilized oil for five months. After the summer growth, the plants were removed from the pots and the roots were separated from the culm., washed to remove the soil. Roots from each cultivar from the sterile and non-sterile environments were subjected to hydrodistillation. Both the cultivars were also grown in posts in sterile and non-sterile plants from plants grown in outdoor plots in Florida. The roots were harvested separated from the culms, washed and subjected to hydrodistillation. All the oil produced were analyzed by GC-FID and GC/MS. Each oil produced was examined for the known 25 constituents as can be seen in T-2. Although the oil yields differed, the yields may relate to the plantlet size rather than only an inherent yield difference.
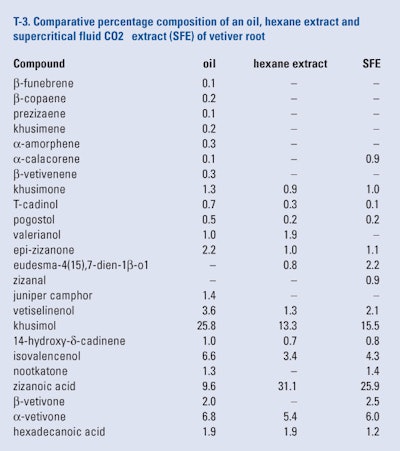
Danh et al. (2009) examined the conditions for the production of a supercritical fluid extract (SFE) of air-dried vetiver roots grown in Australia. They found that the optimum conditions to produce the highest yield were 190 bas, 50°C and extraction time of 100 mins. The authors also produced a 5hr soxhlet hexane extract and a hydro distilled oil from the same batch of dried vetiver roots. The compositions of the oil and exit hexane extract were compared with the SFE using GC-FID and GC/MS as shown in T-3.
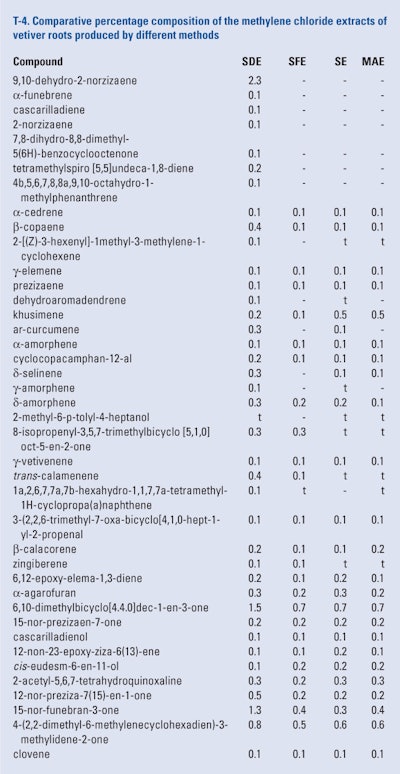
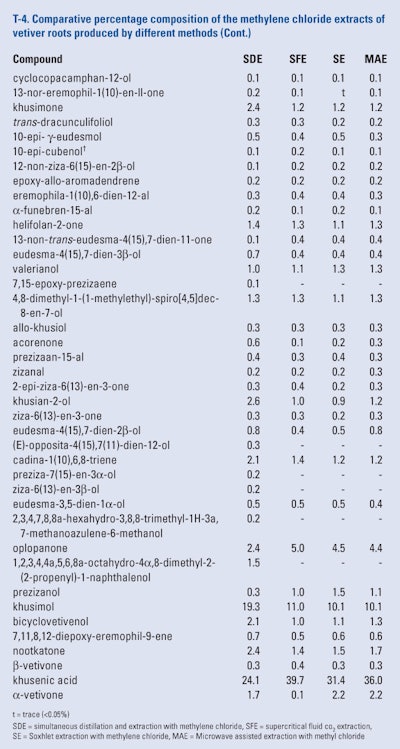
The roots of twelve month-old ‘Mae Hae’ cultivar of vetiver that were harvested, washed, air-dried and ground and blended prior to portions being subjected to simultaneous distillation and extraction using methylene chloride, supercritical fluid Co2 extraction using either 10% methylene chloride, 10% methanol and 0.20% toluene as modifiers, microwave assisted extraction using methylene chloride, methanol or toluene as solvents and Soxhlet extraction using methylene chloride, methanol or toluene as solvent. The extracts were analyzed using GC × GC-FID and GC × GC/MS by Pripdeevech et al. (2010) Because of the large amount of data presented, only the results of the GC × GC analyses of the methylene chloride extracts are presented in T-4.
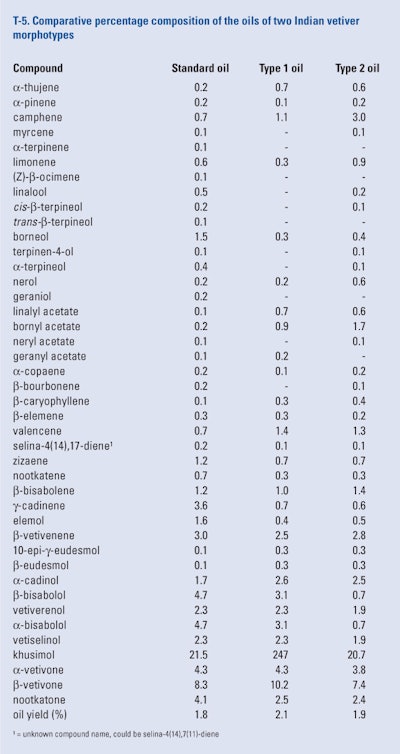
Thara Saraswathi et al. (2011) generated two morphotypes of vetiver from the standard cultivar grown in their experimental garden in Bangalore (India). The two morphotypes produced from the tissue culture of auxiliary buds of the standard cultivar were grown under field conditions for 12 months after which they were harvested. The roots of the standard cultivar and the two morphotypes (long and short) were washed and separately subjected to hydrodistillation. The oils were analyzed using GC-FID, retention indices and co-injection. The results of this study can be seen in T-5.
An oil produced from vetiver roots grown in Adana, the eastern Mediterranean region of Turkey over two seasons was then analyzed by Kirici etal. (2011) using GC/MS only. The components of the two oils, which were reported to be characterized were as follows:
8,9-dehydro-cycloisolongifolene† (0.1–1.8%)
cadina-1(10),4-diene (0.3–1.1%)
ar-curcumene (0.1–2.7%)
δ-cadinene (0.5%)
β-vetivenene (8.2–9.8%)
δ-gurjunene (1.3–2.8%)
ledene oxide† (0.5–1.1%)
himachalene*† (0.1–2.1%)
valencene (0.2–0.3%)
δ-muurolene (1.8–2.9%)
β-eudesmol (0.3–1.1%)
β-guaiene*† (0.4–1.3%)
allo-aromadendrene epoxide (0.3–1.0%)
α-muurolol (2.2–2.6%)
junipenea† (0.4–5.7%)
T-muurolol (2.0–2.4%)
β-caryophyllene† (0.4–3.7%)
cubenol (0.6–0.9%)
caryophyllene oxide (0.5–1.7%)
khusinol (15.7–19.2%)
8-cedren-13-o1† (2.4–2.7%)
β-gurjunene† (3.2–5.2%)
thujopsene† (3.8–6.0%)
dehydro-aromadendrene† (7.3–9.7%)
β-vetivone (2.6%)
α-vetivone (3.2–3.5%)
* correct isomer not identified
† incorrect identification
a also known as longifolene
This report is a typical example of the researcher’s using the computer to identify the constituents of an oil. As can be seen in the above list, many of the identification were incorrect.
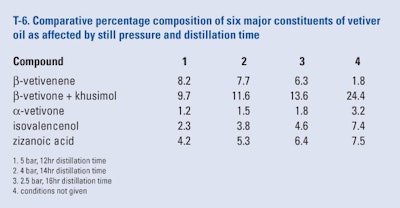
Badie and Beyls (2011) examined the effect of still pressure and distillation time on the content of six major constituents of Indonesian vetiver oil. As can be seen from the results presented in T-6 there were major effects by both pressure and distillation time on the percentage composition of the six selected constituents. Although the best results can be seen from the conditions that were considered to be proprietary, anyone wishing to duplicate these results only needs to perform a series of trials to generate the desired conditions.
Oils produced by hydrodistillation of the roots of two-year-old vetiver plants grown in Bangalore and Kundapur (Karnataka), Hyderabad (Andhra Pradesh) and Mettupalayam (Tamil Nadu) were subjected to analysis using GC-FID and GC/MS by Mallavarapu et al. (2012). The only were found to possess the following range of constituents:
α-cubebene (t–0.1%)
α-ylangene (0.2–0.3%)
α-funebrene (0.1–1.1%)
β-cubebene (0.2–0.3%)
α-cedrene (0.2–0.3%)
β-funebrene (0.2–0.5%)
acora–2,4-dienea (0.2–0.9%)
β-gurjunene (0.4%)
acora-2,4-dieneb (0.2–0.4%)
preziza-7(15)-ene (0.7–1.1%)
ziza-6(13)-ene (1.3–2.0%)
ar-curcumene (1.4–3.7%)
α-vetispirene + cis-eudesma-6,11-diene (1.5–2.1%)
γ-selinene + β-vetispirene (3.9–6.1%)
α-selinene (0.5–0.8%)
γ-amorphene (0.4–1.8%)
sesquicineole (1.2–2.0%)
δ-cadinene (t–0.6%)
nootkatene + γ-cadinene (1.8–2.5%)
δ-vetivenene + cis-calamenene (0–0.1%)
11,12,13-tri-nor-trans-eudesma-5-en-2-one (0.5–1.09%)
α-calacorane (0.3–0.8%)
elemol (0–0.5%)
β-vetivenene (0.8–9.4%)
β-calacorene (1.6–3.7%)
13-nor-eremophile-1(10),6-dien-11-one (0.6–1.2%)
12-nor-preziza-7(15)-en-2-one (0.2–0.3%)
khusimone (1.5–2.1%)
bisabola-1,3,5-trien-7-o1 (0.2–0.3%)
amorph-4-en-10-o1a + 13-nor-eremophila-1 (10)-en-11-one (0.3–0.5%)
10-epi-cubenol + eudesm - 4(15)-en-5β-ol (14–3.4%)
eudesm-6-en-4β-o1 (1.7–2.4%)
1-epi-cubenol (1.0–1.4%)
α-funebren–15–al (0.1–0.4%)
eremophila-1(10),6-dien-12-al (0.9–1.3%)
cyclocopacamphan-12-o1a + cyclocopacamphan-12-o1b + 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-o1 (5.0–6.4%), 3β-methyl-ziza-6(13)-en-3-one + ziza-6(13)-en-12-al (0.6–4.6%)
10-hydroxycalamenene* (0–0.3%)
2α-methyl-2-epi-ziza- 6(13)-en-3-one (2.3–3.2%)
ziza-6(13)-en-3α-ol (0.5–2.4%)
junipercamphorc + (E)-opposita-4(15),7(11)-dien-12-al + isovetiselinenol (1.0–2.4%)
ziza-6(13)-en-3β-o1 (0.7–1.2%)
13-nor-eudesma-4,6-dien-11-one (0.6–0.7%)
vetiselinenol + (E)-opposita-4(15),7(11)-dien-12-o1 (3.9–5.9%)
7,10-epoxy-eremophila-1,9-dien-8α-o1 (0 – 0.3%)
eremophila-1(10), 7-dien-2α-o1 + erimophila-1(10),4(15)-dien-2α-o1 (0.1–0.6%)
nootkatold + khusimol (16.1–19.2%)
6,12,7,11-di-epoxy-eudesm-4-enea (0.3–0.5%)
trans-eudesma-4(15),7-dien-12-yl formate (0.3–0.4%)
6,12,7,11-di-epoxy-eudesm-4-eneb + 7,11,8,12-di-epoxy-eremophil-9-enea (0.2–0.4%)
ziza-6(13)-en-yl formate + (Z)-isovalencenol (0.1–0.4%)
isonootkatol + isovalencenol (5.6–6.9%)
(Z)-eremophila-1(10),7,(11)-dien-12-al (0.7–0.6%)
nootkatone (0.7–1.6%)
β-vetivone (0.8–6.6%)
7,11,8(12)-di-epoxy-eremophil-9-ene + (E)-isovalencenal + trans-eudesma-4(15),7-dien-12-yl acetate (1.0–2.3%)
α-vetivone + khusinic acid (1.9–4.6%)
(E)-eremophila-l(10),7(11)-dien-12-yl formate (0–0.4%)
ziza-6(13)-en-12-yl formate (0.1–0.4%)
(E)-eremophila-1(10),7(11)-dien-12-yl acetate (0.2–0.4%)
* correct isomer not identified
a = epimer A
b = epimer B
c = also known as eudesma-7(11)-en-4α-o1
d = also known as eremophila-1(10),11-dien-2α-o1
The biolarvicidal effect of Indonesian vetiver oil and an ethanolic extract of the distillation root residue was examined by Kadarohman et al. (2013). They found that the ethanolic oxtract of the distillation residue was reasonably effective in controlling the larvae of mosquitoes. Unfortunately, the analysis presented was thwart with errors, so it will not be included in this review.
Soidrou et al. (2013) collected the roots of vetiver from Mchokoujou (Comoros) and subjected them to hydro distillation for 9hrs. Analysis of the oil using GC-FID and GC/MS revealed that it possessed the following composition:
α-phellandrene (0.1%)
(E)-isoeugenol (0.3%)
khusimene (0.5%)
butyl decanoate (0.6%)
α-amorphene (0.3%)
valencene (0.3%)
δ-vetivenene (0.7%)
elemol (0.2%)
β-vetivenene (1.5%)
β-calacorene (0.3%)
spathulenol (0.2%)
trans-sesquisabinene hydrate (0.2%)
khusimone (1.8%)
1,10-epi-cubenol (3.1%)
10-epi-g-eudesmol (0.5%)
1-epi-cubenol (0.5%)
T-cadinol (6.0%)
α-cadinol (2.7%)
7-epi-α-eudesmol (1.6%)
valerianol (1.8%)
epi-zizanone (2.3%)
khusinol (3.0%)
2-khusianol (0.5%)
zizanal (2.7%)
(Z)-β-santalol (1.3%)
nootkatol (3.5%)
juniper camphor (1.0%)
vetiselinenol (1.1%)
khusimol (25.6%)
(E)-β-santalol (0.6%)
14-hydroxy-g-cadinene (0.3%)
13-hydroxyvalencene (1.2%)
bicyclo-vetivenol (11.5%)
isovalencenol (2.2%)
nootkatone (5.3%)
β-vetivone (0.5%)
khusimol acetate (3.8%)
(Z)-β-santalol acetate (2.7%)
α-vetivone (7.8%)
epi-laurenene (0.2%)
A recent review of the volatiles of vetiver by Belhassen et al. (2015) lists the number and structures of constituents characterized in vetiver oil, but it does not include any quantitative date or interpretational data on the accuracy of the data presented.