Authentifying the natural status of flavors is more often than not a challenge that the indutsry faces. When configuring the naturalness of flavor substances, multilevel approaches might offer more reliable results, creating a more transparent supply chain,
In Europe, the naturalness of food flavors is defined legally by regulations, especially European regulation CE 1334/2008. A substance can be considered “natural” when it comes from plant, animal or microbial origin with an enzymatic, microbial or physical treatment.
In this context, biotechnology can be used to obtain natural substances by three possible approaches: through purified enzymes, whole cells (fungi, bacteria, yeasts) or through physical treatments.
Legislation on Flavorings
The aim of the legislation is to ensure the effective functioning of the market by providing a system for regulation of flavors and food ingredients which is harmonized throughout the European Union.a The legislation also aims to ensure a high level of human health and consumer protection, the protection of consumer interests, fair practices in food trade and the protection of the environment. Guidance has been established to clarify issues surrounding flavorings for use in food. Legislative requirements on natural flavors are defined in Regulation 1334/2008, adopted in 2008.
The legislative instrument gives the reference of European Union legislation on natural flavorings currently applicable and lays down the legislative requirements for flavorings and certain food ingredients with flavoring properties for use in and on foods.
The definition of a natural flavoring substance, according to the European regulation, is as follows:
Article 3 (2) (c) - ‘Natural flavoring substance’ shall mean: a flavoring substance obtained by appropriate physical, enzymatic or microbiological processes from material of vegetable, animal or microbiological origin either in the raw state or after processing for human consumption by one or more of the traditional food preparation processes listed in Annex II. Natural flavoring substances correspond to substances that are naturally present and have been identified in nature.
The SNIAA (Syndicat National des Industries Aromatiques Alimentaires), which is the French National Association for Food Flavors Professionals, invites the actors of the industry to guarantee the conformity of the natural flavors through a high level of scientific requirements. As it has been discussed at the SNIAA congress in January 2016 (Isabelle Girod-Quilain, January 14th 2016), multi-criteria visions constitute the basis necessary to ensure compliance with regulation. Naturalness of aromatic substances should be defined with the use of three criteria: the naturalness of the substrate, the natural qualification of the process used to obtain said substances and the analytical authentication of the products involved in this procedure (F-1).
Authentifying the Natural Status of Flavors
From a chemist point of view, a molecule extracted from plants or chemically synthesised in a laboratory is the same if the chemical structure is identical. This introduces an issue in a regulation process: to be applicable, it must be able to clearly differentiate between the same flavoring substance resulting from enzymatic catalysis or chemical synthesis. Furthermore, the interaction of compounds with biological systems has long been shown to be mostly stereo selective. These chiral enantiomers dictate not only the taste or odor quality, but also its intensity or detection threshold.
Authentifying the natural status of flavors is often a challenging problem. Besides GC/MS analysis (identification of by-products), chiral separation of enantiomers and isotope ratio mass spectrometry (IRMS) have been recognized as efficient tools for characterizing the natural or synthetic origin of given chemical species. Commonly in nature, both enantiomers are found even if one isomer is predominant in the mixture. It is very rare to find flavoring substances with an enantiomeric excess of 100%. In the same way, very few, if any, aroma compounds are found to be “racemic”. The European Flavor Association (EFFA)b published its interpretation for optically active flavoring substances in a guidance document in 2015: “mixture of optical isomers shall be allowed in any ratio provided that all the isomers have been identified in nature”.
With this in mind, the determination of enantiomeric ratios provides data to evaluate if chiral compounds are present in a racemic form or not. For many years now the overall content of active components and their relative percentage determined with the use of effective methods were an accepted criterion in the assessment of flavorings [18]. However, this information is not an absolute evidence of naturalness as not all chiral compounds are found in a stable ratio of both enantiomeric forms. As a result, if the analysis of chiral discrimination is a great opportunity for quality control, it also has some limitations. These restrictions are further developed in the following points:
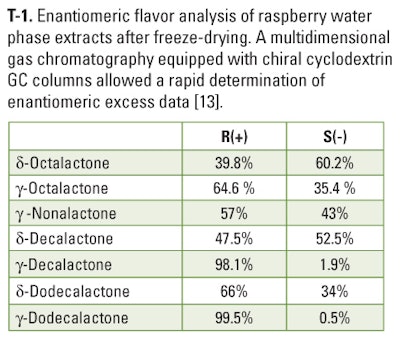
1. Enantiomeric excess can be different in various natural raw materials. Several analytical studies showed that lactones are found with specific enantiomeric distributions depending on the raw material and the molecule considered. In raspberry for example, Dufossé et al (1994-1998) demonstrated that γ-dodecalactone is present with a high enantiomeric excess of the (R) form even if both enantiomers are found in nature. Krammer et al (2006) confirmed these data and extended analysis to the other lactones. T-1 illustrates these results: chiral distribution shows that γ-decalactone occurs in high enantiomeric purities while homologous lactones, such as γ-octa- and γ-nonalactone, are present only in minor enantiomeric purity.
2. During processing or storage a racemization may occur and enantiomers ratios may vary. This can be illustrated with a hazelnut ketone called filbertone. Both enantiomers are found in nature and have a nutty, soft, butter impact however it has been noticed that (+)-(E,R)-Filbertone has an effect about 10 times lower than for (-)-(E,S)-Filbertone. In nature, there is a slight excess of the (E,S)-isomer, the amount varies depending upon the source of hazelnut. The enantiomeric excess in the extracted filbertone is greater under mild processing conditions (T-2).
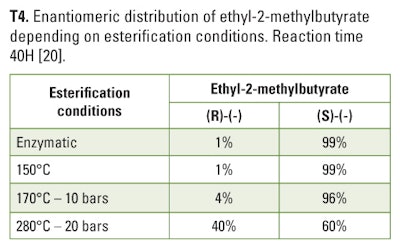
3. Ethyl ester of 2-methylbutyric acid is another substance in which enantiomeric distributions of chiral compounds showed a high excess of the (S)-configuration. This first observation is the main reason which prompted some people to consider racemic ester as artificial even though literature demonstrated a large enantiomeric distribution of the substrate 2-methylbutyric acid in nature due to the numerous possible pathways used by organisms to produce 2-methylbutyric acid (T-3) [14].
On the other hand, esterification of (S)-2-methylbutyric acid with ethanol leads to varying results depending on the conditions applied. It has been demonstrated by Zucca et al (2003) that enzymatic process or soft thermal treatment result in an ethyl ester with a high (S)-configuration excess compatible with natural characteristics [(R)/(S) = 1/99], while a more severe thermic treatment led to racemization of the ester (T-4).
In this case, literature data show that the enantiomeric ratios of ethyl ester obtained by biotechnology or chemical synthesis are not significantly different and cannot be the sole criteria for authentication of natural substances.
In conclusion, the enantiomeric distribution of natural molecules and the analysis of chiral discrimination for authenticity have to be investigated carefully with regard to the interpretation of the natural origin of flavoring substances. Multilevel approaches should be considered for those kinds of flavor compounds to confirm the authenticity of natural substances.
4. Chiral analysis of ionones. One of the most popular aromas in foodstuffs is the fruity compound ionone (F-2). α-Ionone is formed by enantioselective enzyme catalyzed cleavage of carotenoids. It was identified as a flavor component of raspberries, carrots, black tea and violet flowers. The specific aroma of raspberry is formed from carotenoids and has been studied by various authors for many years. Identified in raspberry fruits at first, results showed a nearly strict homochirality with an enantiomeric excess of the R-isomer (99.9%). In order to answer authenticity related questions rapidly, these results have led to the wrong conclusion that the detection of a racemic α-ionone in fruit products shows that a nature identical synthetic compound was added.
Still, there are also reports which show that both chiral forms of α-ionone are found in nature even if the R-form is predominant. Haarmann & Reimer (1991) worked on a variety of foodstuffs to determine the naturally occurring enantiomeric composition of α-ionone in concentrates of violet flowers, fresh raspberries, carrots, vanilla pods as well as absolutes of Osmanthus fragrans and Boronia megastigma. The published results demonstrated that the S-isomer was found at a level of 5% in Darjeeling tea (by solvent extraction) [(R)/(S) = 95/5], 3% in vanilla pods [(R)/(S) = 97/3] and 5% in carrots [(R)/(S) = 95/5] (both by headspace analysis). Boronia absolute has been found to contain 9% of S-isomer and in raspberry also, there were some S-isomer present even if it was found to occur in trace amounts (only 0.1%). These analytical results are in concordance with the natural pathway observed in plants for the synthesis of carotenoids derivative compounds. Literature demonstrates the presence of a large range of carotenoids varieties from which mixtures of α- (R and S) and β-ionones have been produced (F-3).
Carotenoid-derived aroma compounds are important flavor substances in the scent of fruit and flowers. These compounds are formed in three steps: the oxidative cleavage by carotenoid oxygenases, the enzymatic transformation to polar intermediates, and the acid catalyzed conversion into volatiles (F-4).
Over the last years, the number of publications on carotenoid cleavage enzymes (subsequently also named as carotenases – STEP I, F-3), have increased rapidly. These enzymes are isolated from natural sources, for example from nectarine peel (Prunus persica var. nucipersica), flower petals of Osmanthus (Osmanthus fragrans var. aurantiacus), or Japanese Green Tea (Camellia sinensis) and volatile compounds have been investigated by many research groups [1].
Osmanthus fragrans is a shrub native to East-Asia belonging to the Oleaceae family and shows, by far, the highest diversity of carotenoid derived aroma compounds among the flowering plants. Among the over 100 identified volatiles, β-ionone, α-ionone, dihydro-β-ionone, dihydro-β-ionol, and cis- and trans-theaspirane are major components (F-5) [9; 10].
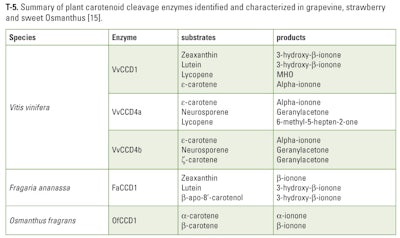
At the same time, Lashbrooke et al identified, isolated and characterized grapevine CCDs (Carotenoid Cleavage Dehydrogenase) involved in flavor and aroma related to apocarotenoid production. They elucidated the biological role of these genes and its respective enzymes. Their study reports a summary of plant CCDs identified in literature, including grapevine enzymes, with cleavage sites, substrates and products showing the presence of CCDs specific to ε-Carotene. ε-Carotene splits on the cleavage site 9, 10 (9', 10') and leads directly to the formation of α-ionone (R and S) (T-5).
Plant CCDs have been shown to be involved in the continuous synthesis and degradation of carotenoids and chlorophyll. These mechanisms are of critical importance in the maintenance and regulation of active photosynthesis and for adaptation to changing light conditions. Depending on its localization, CDDs have various substrate specificities (T-3: VvCCD1 localized in the cytosol; VvCCD4a and VvCCD4b localized in the chloroplast). This allows considering the possibility to produce, by bioconversion, a large range of carotenoids derivatives and in particular both enantiomers of a-ionone with specific ratios.
Based on this conclusion, (R)-α-ionone pure enantiomer biosynthesis has been realized by genetic engineering. Katrin Schullehner (Phytowelt Green Technologies GmbH, Germany) shared her studies on biosynthetic routes for ionone biosynthesis during the Bioflavor International Conference in Frankfurt (Sept. 2015).
To biosynthesize enantiomeric pure (R)-alpha-ionone, Schullehner et al engineered a strain of E. coli and proposed a pathway via the synthesis of ε-carotene and then its cleavage to (R)-α-ionone (F-6). To do so, Phytowelt Green Technologies GmbH had to engineer a specific lycopene epsilon cyclase to optimize the carotenoid pathway (wild type enzyme screening / directed evolution / clones screening) and further used a carotene cleavage enzyme CCD to convert it to enantiomeric pure (R)-α-ionone. This CCD was isolated from plant with a characterized activity on ε-carotene and led to the biosynthesis of the (R) pure candidate enantiomer.
On the other hand, J. Lopez [16] presented a work on the production of β-ionone by combined expression of carotenogenic and plant CCD1 genes in Saccharomyces cerevisiae. Integration of various genes from the carotenoid pathway resulted in carotenoid producing cells. Lycopene is then transformed via a lycopene beta-cyclase widely present in the plant kingdom (F-7). Various vectors or cloning strategies were tried and resulted in a final 0.63 ± 0.02 mg/g of β-ionone concentration. Batch fermentations with this strain resulted in a final specific concentration of 1 mg/g in 50 h. In this case, a β-ionone producing yeast platform has been constructed.
These studies prove that with an enlightened choice of enzymes used for the bioconversion, a chiral ratio specification can be set up. In these cases, chiral analyses are not sufficient to answer authenticity related questions. Natural flavoring substances are often showing high complexity which requires the application of combined analysis to guarantee the naturalness of the product on a strict scientific point of view.
New Challenges and New Solutions
The human organism is chiral and chiral reception is the primary criterion in biological activity. Metabolic processes regulated by biological molecules are sensitive to stereochemistry and different reactions can be observed when comparing the activity of pairs of enantiomers.
Aromatic substances present in foodstuff are submitted to the same law and aroma perception depends on the effectiveness of interactions between the gustatory receptors and the flavor compounds. As a consequence, it is often believed that nature provides pure enantiomers of chiral flavor compounds whereas it produces various ratios of stereoisomers with the predominance of one enantiomer. For many years now, the chiro-specific differentiation of optically active flavoring substances and the measurement of its relative percentages have created a powerful tool in the assessment of natural flavors. However, for some chiral flavor compounds, there is a wide variation of enantiomeric distribution in nature. In such case, the assessment of their naturalness has to be done by a combination of techniques, including but not limited to chiral analysis.
In this context, issues surrounding chirality and natural authentication in food flavors are a good example to describe the new challenges for flavor and fragrance companies for analytical quality control and performance analysis. Any test method has its limitations with regard to accuracy, precision, sensitivity, linearity, reproducibility and specificity. Flavoring substances are often showing high complexity, which requires the application of combined analytical methods. Besides chiral separation of enantiomers, GC/MS analysis and isotope ratio mass spectrometry (IRMS) are receiving increased attention. Authentication of a natural flavoring substance can also be done through a comprehensive knowledge of the production process compatible with EU regulation.
Since it is known that both enantiomers of a-ionone occur in many raw material containing carotenoids, a detailed analysis of these qualities is necessary and the chiro-specific analysis should be completed by other data. Using a metabolic engineering approach, we have shown that it is possible to produce ionones with specific chiral ratio, depending on the enzymes chosen in the biosynthetic pathway. In addition, European Regulation EC 1334/2008 defined precisely the “natural” definition of flavoring substances. It allows the use of physical, enzymatic and microbiological treatments. These processes lead to the formation of natural molecules with analytical characteristics specific to the process used to produce them and not derived from aroma materials extracted from plants. Cutting-edge analytical tools are essential to assess authenticity of natural flavoring substances, but these studies must nevertheless be complemented with process scrutiny and auditing of the manufacturing premises.
Acknowledgements
We would like to thank Eric Angelini for his expert advice on natural regulation.