Widely used in the perfume industry, cis4Isopropylcyklohexylmethanol (CAS#: 13828370), commercially known as Mayola, exhibits a fresh scent combined with floral traces of many flowers such as white petals, blossoms, magnolia, tuberose, etc.1,2. Though 4isopropylcyklohexylmethanol is a stereoisomeric molecule, its isopropyl group can have either cis or trans configurations. For commercial and general purposes, these isomers are used as a mixture and have almost the same organoleptic properties as individual chemical compounds.3 The certain mixtures are defined by the ratio of the isomers, usually in favor to the cis-isomer (Mayol). In combination with woody fragrances, such as methylionone, Mayol spreads the woody scent.2 Mayol is used in a very wide range of cosmetics, toiletries and household products. Its tenacity on a smelling strip is three days, and its typical use in recommended applications is 0.55 %.2 The worldwide use of Mayol is about 10,100 metric tons per year.4
Preparation of 4isopropylcyklohexylmethanol comes out commonly from β-pinene in reaction with peracetic acid over Raney nickel.1 Another way is to use acetic anhydride and acetic acid as an acid catalyst reacting with β-pinene oxide.5 β-Pinene or β-pinene oxide can be used as a substrate for perillyl alcohol preparation, which can be used as a precursor for Mayol.6 4-Isopropylcyclohexylmethanol can also be selectively prepared from cuminaldehyde by hydrogenation using 5% Ru/supported commercial catalyst. 7
This work was focused on preparation of Mayol from cuminaldehyde (4(1methylethyl)benzaldehyde) using cheaper Ni supported catalysts. Mayol preparation from cuminaldehyde over a nickel supported catalyst consists of several consequential steps in comparison to the preparation catalyzed by Ru catalyst (F-1). Preparation over a nickel catalyst is considered to be low-priced, although there are significantly more demanding steps in the whole process.
Two ways for the preparation of Mayol from cuminaldehyde exist that differ in the protection type of aldehydic group. The first way consists of direct protection of aldehydic group by acetalization. The second one is the transformation of cuminaldehyde to cuminic acid (VI) and formation of ester (VII).
The first way involves the protection of the carbonyl group of cuminaldehyde by e.g. acetalization or by the formation of cyclic acetals.8 The protection of the carbonyl group can also be performed using disulphides9 or tertbutylamine.10 1,3Dioxanes and 1,3dioxolanes (VI) can easily be prepared from carbonyl compounds in reaction with ethane-1,2-diol (ethylene glycol) in the presence of acid catalyst.11 Deprotection is often performed by acid-catalyzed transacetalization in acetone or hydrolysis in wet solvents or in aqueous acid.
The other way starts by oxidation of cuminaldehyde to cuminic acid (VI). This can be prepared from cuminaldehyde by oxidation using hydrogen peroxide or by the Cannizzaro reaction. In the latter reaction cuminic alcohol is obtained as a by-product and this by-product can also be converted to acid 12 or can be directly hydrogenated to Mayol using e.g. Ru catalyst.7

The next step is based on azeotropic esterification of cuminic acid by butan-1ol in presence of acidic catalyst. The aromatic ring of the prepared cuminic acid butyl ester (VII) can be hydrogenated over nickel supported catalyst. The final step is the deprotection of protected carboxylic group and the formation of desired saturated alcohol – Mayol. In the case of butyl ester, the direct formation of Mayol is possible due to the hydrogenolysis using Adkins catalyst.
Many research works have been published on the topic of Mayol preparation from various substrates. But the overall problems of its preparation from cuminaldehyde when using Ni catalysts has not been previously published. Even though many steps are involved, the high yield and the purity of the final product may overshadow the possible disadvantages. We would like to show how to obtain the mixture of isomers of cis4isopropylcyklohexylmethanol from cuminaldehyde in four-step synthesis with specific aim on the test of commercial Ni supported catalysts in the hydrogenation step and Adkins catalysts in hydrogenolytic step.
Experimental Cannizzaro Reaction
The preparation of cuminic acid has been carried out in a magnetically stirred triple-neck flask with a thermocouple and dropping funnel attached. In a typical experiment 49.2 g cuminaldehyde (Givaudan SA; p.a.) was put into the flask and a solution of 32 g potassium hydroxide (Lachner; p.a.) in water (30 ml) was added drop wise. After the increase of density, 27 ml of butan-1-ol (Penta; p.a.) was added. The reaction mixture was intensively stirred for 6.5 hours in temperature ranged from 60 to 70 °C. Afterwards hydrochloric acid (Chemapol; p.a.) was introduced into the mixture to reach pH 4. The mixture changed color from orange to yellow. Next, 25 ml of water was added, and organic and water phases were separated. The water phase was washed with 10 ml of butan-1-ol and the organic phases were gathered.
Esterification
For the preparation of cuminic acid butyl ester, prepared cuminic acid, butan-1-ol (40 g) and ptoluenesulfonic acid (PTSA) (Chemapol; p.a.) as an acidic catalyst (0.3 g) were put into a magnetically stirred triple-neck flask. The composition of the azeotrope with b.p. 92.7 °C at normal pressure is 44.5 wt. % of water and 55.5 wt. % of butan-1-ol. The mixture was stirred for 10 hours until no water was separated in Dean-Stark apparatus and then cooled down. Afterwards, 12 ml of saturated water solution of sodium hydrogen carbonate (Penta; p.a.) was added in the reaction mixture. The organic and water phases were separated. The organic phase was furthermore washed with water (30 ml), and the water phase was washed with butan-1-ol. The organic phases were gathered and distilled.
Carbonyl Group Protection
The carbonyl group protection has been performed by acetalization with ethane-1,2-diol.
In a typical experiment, 74 g of cuminaldehyde, 62 g of ethane-1,2-diol (Chemapol; p.a.), 0.125 g of PTSA and 200 ml of cyclohexane (Penta; p.a.) were put into a magnetically stirred triple-neck flask with a thermometer and Dean-Stark apparatus. The composition of the azeotrope with b.p. 69.8°C at normal pressure is 91.5 wt. % of cyclohexane and 8.5 wt. % of water. The mixture was stirred for 4 hours. Afterwards the solvent was evaporated and the product (2pcumenyl1,3dioxolane) was refined by vacuum distillation.
Hydrogenation
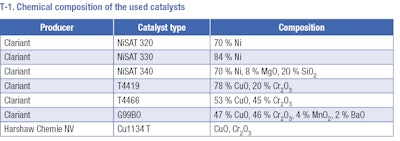
Hydrogenation experiments were carried out in a stainless-steel autoclave. The selected amount of substrate, 100 ml of butan-1-ol (if the solvent was used) and 20 wt. % of the catalyst was inserted into the reactor. Commercially available Ni supported catalysts (T-1) were tested in hydrogenation of the aromatic ring under various conditions. Several types of CuCr catalysts (T-1) were tested in hydrogenolysis of butyl ester of pisopropylcyclohexane carboxylic acid to Mayol. The autoclave was washed by hydrogen and heated to the desired reaction temperature. After heating, the reactor was pressurized, and the reaction was started by switching on the stirring. Samples taken during the reaction were analyzed on a GC equipped with an FID and non-polar column. Identification of individual chemical compounds has been performed by GC-MS.
The reaction mixture was filtrated over a diatomaceous earth filter and the solvent was evaporated. The final product was then refined by vacuum distillation.
Results and Discussion
Mayol was prepared by multi-step synthesis from cuminaldehyde using Ni supported catalysts in the hydrogenation step and Adkins catalysts in the hydrogenolytic step. Direct hydrogenation of cuminaldehyde over Ni supported catalysts is not possible due to the strong hydrogenolytic properties of this metal under conditions that are necessary for hydrogenation of a benzene ring.
Direct hydrogenation of cuminaldehyde
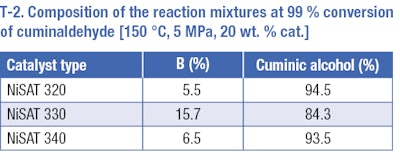
We confirmed this by testing direct hydrogenation of cuminaldehyde over nickel catalysts at a temperature of 150 °C and pressure of ٥ MPa (T-2). The undesirable cleavage of cuminaldehyde to 1-methyl-4-(propan-2-yl)benzene (p-cymene) occurs. The amount of p-cymene was relatively high especially in the case of NiSATb 330 (catalyst with the highest amount of Ni). Moreover, at total conversion of cuminaldehyde, no desired saturated alcohol was detected in the reaction mixture and cuminic alcohol was the main product of the reaction, meaning that the benzene ring remained in the reaction mixture under studied conditions. When performing the reaction for a longer time and under more severe conditions, only hydrogenolysis producing p-cymene was observed.
Carbonyl Group Protection Using Acetalization – Hydrogenation
The second way of how to use cuminaldehyde for Mayol preparation is by protection of the carbonyl group with ethane-1,2-diol. 2pCumenyl1,3dioxolane (II) has been prepared from cuminaldehyde by acetalization with ethane-1,2-diol catalyzed by PTSA (F-2). The product was separated by vacuum distillation under a pressure of 4.66 kPa (bp 141155 °C). The reaction yield was 84% (77 g) with 95% purity.
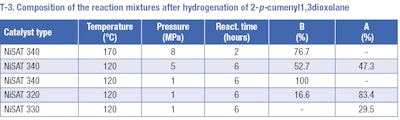
The product, 2pcumenyl1,3dioxolane (II), may be used for hydrogenation of the aromatic ring, however this method has been discovered to be also unusable. Several hydrogenation reactions were carried out at temperature range of 120 to 170°C and a pressure range of 1 to 8 MPa over nickel catalysts (T-3). Preferably, the cleavage of the C-O bond appears rather than hydrogenation of the aromatic ring. The formation of 2[(4isopropylphenyl)methoxy]ethanol (A) and 1-methyl-4-(propan-2-yl)benzene (B) afterwards occurs for all used catalysts. No desired 2pisopropylcyclohexyl1,3dioxolane (III) was detected in the reaction mixture.
Cannizzaro Protection by Esterification – Hydrogenation – Hydrogenolysis
The protection of the carbonyl group was at first by esterification of cuminic acid obtained via the Cannizzaro reaction of cuminaldehyde. The Cannizzaro reaction (described in the experimental section) was performed and 25.5 g of cuminic acid was obtained (yield 93.5%) to be used in esterification. This step is necessary due to the simple elution of Ni from the supported catalyst in the presence of acids (even weak ones). Azeotropic esterification of cuminic acid by butanol using PTSA as a catalyst yielded to the desired butyl ester. After vacuum distillation at a pressure of 0.53 kPa (b.p. 115122 °C) 28 g (82 % yield) of thr desired product, 94 % purity was obtained.
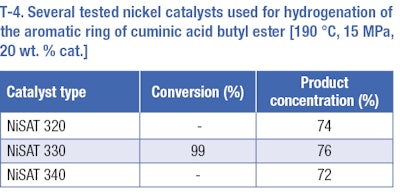
The same catalysts that were used for the hydrogenation of cuminaldehyde were used for hydrogenation of the benzene ring of butyl ester of cuminic acid. Hydrogenation reactions were carried out under more severe conditions, at a temperature range of 170 to 190°C and a pressure range of 10 to 15 MPa. The increase of temperature and pressure had a positive effect on the selectivity of the desired product. A temperature of 190°C and a pressure of 15 MPa—based on results of previous tests—were used to compare various nickel catalysts (T-4). The best results for hydrogenation of the aromatic ring were obtained at 190°C, 15 MPa, 20 wt. % Ni-supported catalyst NiSAT 330, but the results of all catalysts differed in the range of measurement error. The reaction yield of such a reaction was 76% (of cis/trans4isopropylcyclohexane carboxylic acid butyl ester) at total conversion. The rest consists of undesirable products, e.g. 1-methyl-4-(propan-2-yl)benzene, 1methyl4isopropylcyclohexane (p-menthane).
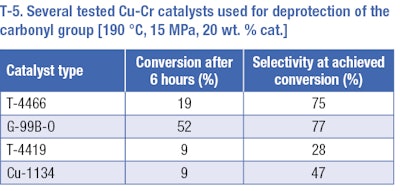
Hydrogenation of the aromatic ring of the protected substance provided positive results. The following experiments were focused on deprotection of pisopropylcyclohexane carboxylic acid butyl ester to Mayol. Several reactions at a temperature of 190°C and pressure of 15 MPa were carried out. Four types of catalysts were tested at these conditions (T-5). The best results (selectivity 77% and conversion rate 52% after 6 hours) were achieved on the G-99B-0 Pulver catalyst – the catalyst with BaO and MnO2 additives. The comparable selectivity was obtained using T-4466 catalysts (a comparable amount of copper and chromium oxides, without additives) but under lower conversion. Unsatisfactory results were obtained using the other two catalysts. Low selectivities under low conversions, p-cymene and p-menthane occurred as by-products.
Large-scaled experiment
On the basis of the previous experiments, a larger scaled experiment was performed starting from cuminic acid (Sigma-Aldrich; purity ≥98%). Cuminic acid butyl ester was prepared by azeotropic esterification using cuminic acid, ptoluenesulfonic acid and butan-1-ol. The initial feed was cuminic acid (102.8g), butan-1-ol (250 ml) and PTSA (1.2g). The product was then separated by vacuum distillation under a pressure of 0.27 kPa (b.p. 125, 130°C). The reaction yield was 88% (122g) with 98٪ purity. Cuminic acid butyl ester was then hydrogenated over Ni-supported catalyst NiSAT 330 at a temperature of 190°C and a pressure of 15 MPa. The conversion of the reaction was 97% after 19 hours. The reaction mixture containing 82.4% of both isomers of pisopropylcyclohexane carboxylic acid butyl ester was then treated over G-99B-0 Pulver catalyst (F-3) without any purification. The achieved selectivity was 87% at total conversion after 33 hours. The final product (4isopropylcyclohexylmethanol) was separated by distillation. A mixture of cis/trans4isopropylcyclohexylmethanol 60:40 with 99% purity (sum of both isomers) was obtained in a 40% yield related on cuminic acid. Regarding the multi-step process, the yield is satisfactory.
Conclusion
The preparation of p-isopropylcyclohexylmethanol—its cis isomer also called Mayol—from cuminaldehyde was performed. Neither direct hydrogenation of cuminaldehyde nor cuminaldehyde protection by acetalization with ethane-1,2-diol followed by hydrogenation over a nickel catalyst led to a desirable product.
The optimal way is to convert cuminaldehyde to cuminic acid by the Cannizzaro reaction, and protect the carboxylic group by esterification with butan-1-ol catalyzed by PTSA. The prepared cuminic acid butyl ester was hydrogenate over nickel. Performing these steps using optimal conditions (190°C and 15 MPa) and catalyst NiSAT 330 (20 wt. %), the yield of pisopropylcyclohexane carboxylic acid butyl ester was 85%.
The prepared pisopropylcyclohexane carboxylic acid butyl ester was hydrogenolyzed over CuCr G-99B-0 Pulver catalyst at 190°C, 15 MPa with 87% selectivity at total conversion. Both cis/trans4isopropylcyklohexylmethanol isomers were formed in a ratio of 60:40. For the first time the desired synthesis of Mayol from cuminaldehyde was performed over a nickel catalyst.
4Isopropylcyklohexylmethanol was obtained as a final product of large-scaled experiment in 40% yield (99% purity). This widely used fragrance was also subjected to analytical and sensorial evaluation. The received results were comparable with the commercial standards.