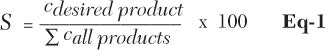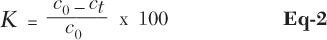
1-(4-Isopropylcyclohexyl)ethanola is a natural-identic substance appearing as clear, colorless to pale yellow liquid with a light, floral and muguet note.1 Due to its stability, the material finds use in a large number of products in the fragrance industry. It has four diastereoisomers, with (-)-1-S-cis-isomer having the most intense lily-of-the-valley scent, although the others may be used as well.2-4 1-(4-Isopropylcyclohexyl)ethanol (F-1) may be most simply prepared from cuminone (4-isopropylacetophenone) by hydrogenation. Cuminone itself is a colorless liquid and is also used in flavor and fragrance chemistry.2 Its iris and basil scent is included in the group of warm, spicy wood aromas. It can be prepared by Friedel-Crafts acylation of cumene by acetyl chloride or acetic anhydride.5, 6
Cumene is relatively cheap and readily available, as it is manufactured in large amounts for the large scale production of phenols. Friedel-Crafts acylation represents a useful reaction in fine chemical synthesis. Traditional catalysts used for this reaction are hazardous soluble Lewis acids, e.g., AlCl3, TiCl4 and FeCl3, or strong mineral acids, such as hydrofluoric (HF) that increase the production of waste and are destroyed during the reaction.7-10 In order to decrease the problems caused by the use of such catalysts, alternative catalysts were developed. These include lanthanide triflates11, 12 and very important groups of solid Lewis acids, e.g., FeCl3 over K10,13 clay catalysts14 and solid acids15, 6 including zeolites, especially zeolite ZSM-5 and Beta.5, 6-23 The acylation of cumene by acetyl chloride and acetic anhydride has been referenced in a limited number of articles.5, 6, 24-27 Further, the hydrogenation of cuminone to 1-(4-Isopropylcyclohexyl)ethanol is described only chemoenzymaticaly,4 where the optimization of classical three-phase hydrogenation is missing. Thus, the preparation of 1-(4-Isopropylcyclohexyl)ethanol from cumene illustrates an interesting and simple way to obtain a fine chemical from a commodity.
Presented here is the two-step preparation of 1-(4-Isopropylcyclohexyl)ethanol starting from cumene. In the acylation step, both acetic anhydride and acetyl chloride were used. For the hydrogenation step, Ru-supported catalysts were used—taken from the authors’ experience from the hydrogenation of cuminic aldehyde.28
Materials and Methods
Acylation: In a typical experiment using anhydrous AlCl3b (2.5 g) or FeCl3c (3.1 g) as a catalyst, calculated amounts of cumened (17.9. g) and acetic anhydride (AA)c (1.9 g) or acetyl chloride (AC)e (2.15 g) were inserted to a round-bottomed flask, together with dodecane as an internal standard (1.9 g).e This and a separate flask, containing the catalyst, were cooled to the desired temperature (-5°C – 5°C). The contents of both flasks were then mixed, and the reaction started after precooling. The samples from this reaction mixture were treated with water and neutralized by Na2CO3.
Zeolitesf, g (see T-1) were activated before the acylation reaction by calcination at 150°C for at least 2 hr under nitrogen atmosphere. To these preactivated catalysts, the cumene/acetic anhydride or acetyl chloride/internal standard reaction mixture was added under stirring. These samples were centrifuged and analyzed.
Hydrogenation: Hydrogenation of prepared 4-isopropylacetophenone (cuminone) was performed in a batch autoclaveh. In a typical experiment, 10 g of cuminone in 90 mL of the solvent isopropyl alcohol were inserted to the autoclave with 5% w/w catalyst, typically 5% Ru/C (see T-2), calculated on the amount of cuminone. The reactor was pressurized three times to 2 MPa with hydrogen, emptied, heated to the desired temperature (130°C) and pressurized (5 MPa).
All samples were analyzed by gas chromatographyj with flame ionization detection (FID) using a non-polar capillary columnk. Zeolites were characterized to confirm their Si/Al ratio using x-ray fluorescence and a spectrophotometerm.
Calculations
For the evaluation of reactions, the following values were evaluated: selectivity (Eq-1), conversion (Eq-2) and yield (Eq-3).
Here, S is selectivity in % and c is the concentration of products in the reaction mixture.
Here, K is conversion in %, co is the concentration of acylating agent at the beginning of the reaction and ct is the immediate concentration of acylating agent.
Here, Y is yield of the reaction in %, mr is amount of product in the reaction mixture calculated using the internal standard, and mt is the theoretical yield of the product calculated from the chemical equation.
Acylation Results: Classic Lewis Acids
Since the best acylation reaction yields are obtained using classical Lewis acids, i.e., anhydrous AlCl3 and FeCl3, these catalysts were used to prepare the cuminone. A comparison was made using these catalysts and the two acylating agents. The highest yield (98%) was obtained using AlCl3 and acetyl chloride at 0°C. Here, the molar ratio of cumene/acetyl chloride was 6:1, and the molar ratio of acetyl chloride/catalyst was 1. A comparable yield (96%) was obtained using FeCl3 but at room temperature. The use of higher temperatures with FeCl3 compared with lower temperatures using AlCl3 is also confirmed in the literature.29
Lower yields (up to 86%) were obtained when acetic anhydride was used as an acylating agent, FeCl3 was the catalyst, the molar ratio of cumene/acetic anhydride was 6, and the molar ratio of acetic anhydride/catalyst was 1. This corresponds to the lower activity of anhydride, compared with chloride. In spite of the lower yield, however, the use of anhydride was preferred due to its simpler manipulation and lower toxicity. Although the literature10 suggests a molar ratio of acylating agent to catalyst at 1:3, comparable results were obtained using a ratio 1:1, which is better for the environment.
The reaction yield was influenced by the formation of side products, namely 2,3-dimethyl-2,3-diphenylbutane (F-2a), 4,4´-diisopropylbiphenyl (F-2b) and 1,3-diphenylbutan-1-one (F-2c).
Acylation Results: Zeolites
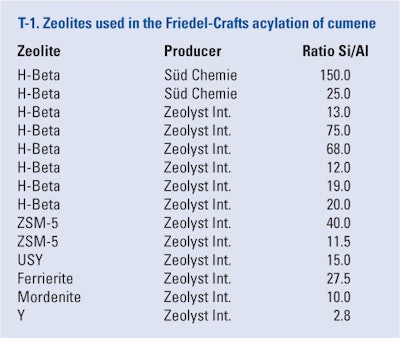
The literature10, 30 suggests acetic anhydride be used as the acylating agent when zeolites are used as the catalysts. The authors also tested acetyl chloride but its yield only reached up to 9%. Thus, to test zeolites in the Friedel-Crafts acylation of cumene, acetic anhydride was chosen. The most commonly used zeolites for this type of reaction are beta zeolites. These catalysts are typified by the presence of Broensted acid centers in micropores and on the outer surface of the catalyst, and Lewis acid centers, which are present on the inner surface.31 Beta zeolites differ in their ratio of Si/Al, which also determines the acidity of the catalyst; with increasing Si/Al ratio, the acidity of the catalyst decreases and hydrophobicity increases. The present experiments were carried out using a molar ratio of cumene/acetic anhydride = 6, a weight ratio of acetic anhydride/zeolite = 1, and at a temperature of 150°C. The results are summarized in T-3. In almost all cases, the maximal conversion and selectivity was reached at approximately 3 hr, except in the cases of zeolites beta 12 and 13, where maximal conversion was reached at 1 hr and 2 hr, respectively. The reactions were carried out up to 25 hr but the conversion did not change and the selectivity decreased. Lower levels of conversion may be attributed to catalyst deactivation by the products of the reaction.
From T-3 three facts are clear:
1. The yield of the reactions using zeolites was lower compared with classic Lewis acids. This was due to several reasons, especially the deactivation of zeolite catalysts.
2. The selectivity of the reaction also was lower. This was due to the presence of Broensted and Lewis acid centers close in proximity to one another on the catalyst and partially by shape selectivity. In all cases, the major product was the desired cuminone-4-isopropylacetophenone; although when zeolites were used, 2-isopropylacetophenone was also observed in the reaction mixture.
3. The only zeolite catalysts suitable for acylation were the beta zeolites, where the yield decreased with an increasing Si/Al ratio, i.e., with the decreasing acidity of the catalyst. The highest yield (88%) was obtained using zeolite beta Si/Al ratio 12.
Even though zeolites Y and USY belong to the group of large-pore zeolites, the yield obtained using these catalysts did not reach 10%. This was probably caused by their deactivation during the reaction. Zeolite ZSM-5 is usually used for the acylation of less substituted aromatic molecules, i.e., benzene, phenol and toluene,19 and the low yield obtained in this work confirmed it was unfit for cumene acylation.
Acylation Results: Zeolites and Reaction Optimization
Reaction conditions were optimized using zeolite beta (12). Here, the molar ratio of C/AA and temperature were tested, and their influence on selectivity and yield were evaluated (T-4). Despite the fact that the literature19 offers an optimal ratio of toluene/AA = 20, negative influences of such high ratios on the reaction rate and conversion were detected. These negative influences included higher amounts of cumene in the reaction mixture, which may be explained by the increased formation of by-products or cumene itself blocking the active centers of zeolite, which is a relatively large molecule. The author found the optimal molar ratio of C/AA to be = 6.
Optimization of the temperature could only be performed in a small range because at temperatures lower than 130°C the reaction does not take place; further, the boiling point of the mixture is under 155°C. Thus, optimization was performed using temperatures of 130°C, 150°C and reflux. With increasing temperatures, the reaction rate increased significantly—as did the total conversion of AA. The selectivity remained the same, at around 89%.
Note the undesired products from this reaction mixture were totally different than those produced by the classic Lewis acids. When zeolites were used as catalysts, 2-isopropylacetophenone (F-3a), 3-methyl-4-phenylbut-3-en-2-one (F-3b) and 2-phenylpent-2-en-4-one (F-3c) were detected.
Cuminone was thus prepared on a larger scale using FeCl3 as a catalyst—at room temperature, with a molar ratio of cumene/acetic anhydride = 6, with molar ratio of catalyst/AA = 1, and distilled under a reduced pressure of 10 mbarr at 115°C. This produced cuminone having 99.5% purity, which was used for the hydrogenation experiments. As a typical first step, cuminone (F-4) initially was hydrogenated to (4-isopropylcyclohexyl)methyl ketone (two isomers, cis and trans) or 1-(4-isopropylphenyl)ethanol. Both of these products were hydrogenated to the desired 1-(4-isopropylcyclohexyl)ethanol. In the reaction mixture, the product was present in cis and trans isomer forms at an approximate ratio of 72:28. The selectivity of the product was calculated on the sum of both isomers. The typical reaction course is depicted in F-5.
The only undesired product detected in the reaction mixture was 1-ethyl-4-isopropylcyclohexane, which is formed by hydrodeoxygenation especially of (4-Isopropylcyclohexyl)methyl ketone. Its unsaturated analogue, p-ethylcumene, was not detected. Confirming the fact that carbonyl compounds are less easily hydrogenated than other unsaturated bonds, larger amounts of saturated ketone ((4-Isopropylcyclohexyl)methyl ketone) were found in the reaction mixture, compared with unsaturated alcohol (1-(4-Isopropylphenyl)ethanol) (see F-5). From the reaction course, the fast consumption of cuminone was visibly connected with the fast formation of the products. At the end of the reaction, only the desired 1-(4-Isopropylcyclohexyl)ethanol, together with 1-ethyl-4-isopropylcyclohexane, was present.
Hydrogenation Results
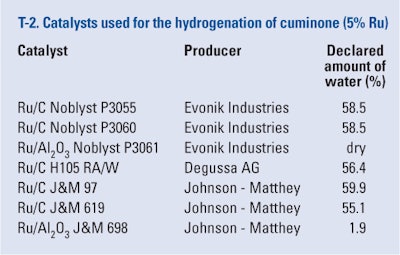
The effects of several commercial Ru/supported catalysts on hydrogenation were tested. These catalysts were compared regarding selectivity and reaction rate. The results are summarized in T-5. The selectivity was calculated at total conversion of cuminone and intermediates from Eq-1 and the reaction rate was calculated in 30th minute of reaction, per Eq-4.
Here, no is initial amount of cuminone, n30 is amount of cuminone in 30th minute of reaction, mcat is amount of catalyst (calculated dry) and t is time (30 min). The reaction conditions were chosen based on previous measurements: 130°C,28 since below this temperature, the hydrogenation of the benzene ring runs slowly and above this temperature, hydrodeoxygenation is more probable; and 5 MPa for equilibrium reasons.
From T-5, it is obvious that the selectivity to 1-(4-Isopropylcyclohexyl)ethanol strongly depends on the catalyst type. From the catalysts produced by Evonik (Noblyst), the only one with high selectivity was Noblyst P3061, which is 5% Ru/alumina; its use was also characterized by the lowest reaction rate. The other two catalysts, also 5% Ru/C, produced a high amount of 1-ethyl-4-isopropylcyclohexane in the reaction mixture. The least favorable for selectivity under the chosen conditions was the catalyst from Degussa. All catalysts from Johnson Matthey
possessed comparable selectivity, ranging from 86.5 to 91.3. The reaction rates also were comparable. It can thus be concluded that selectivity is: dependent on the catalyst type and independent of the support and amount of water in the reaction mixture, per supplier declarations and internal measurements. In all cases, the desired 1-(4-Isopropylcyclohexyl)ethanol was the main product, with a similar ratio of cis and trans isomers. The optimization of reaction conditions may be advisable, but the high selectivities are very promising.
Conclusions
An important fine chemical for the industry, 1-(4-Isopropylcyclohexyl)ethanol, was prepared from the cheap and available commodity cumene by a two-step synthesis process.
In the first step, the acylation of cumene by acetyl chloride and acetyl anhydride was performed using two types of catalysts: classic Lewis acids and zeolites. These catalysts, along with the acylating agents, were compared and the following conclusions were formed: Acetic anhydride may be preferred as an acylating agent due to its stability, availability and the limited waste produced, i.e., only acetic acid. Comparing catalysts, the classic Lewis acids provided higher yields of cuminone but also higher waste levels. When using zeolites, especially zeolite beta (12), the yields were lower but the only problem they posed was regeneration, because the catalyst is quickly deactivated during the process.
The hydrogenation of cuminone, the second step in 1-(4-Isopropylcyclohexyl)ethanol preparation, was a very selective reaction, with the only undesired product, 1-ethyl-4-isopropylcyclohexane, being formed by hydrodeoxygenation of the intermediate. For hydrogenation, Ru supported catalysts may be suggested. The selectivity was about 90% and 4-isopropylcyclohexyl)ethanol was obtained in two isomeric forms, with a ratio of cis:trans at 72:28 (mass). The overall yield of the reaction was 75% calculated on cumene with purity of the product 99.5%. This result is very promising for the industrial application, as it shows it is possible to prepare valuable substances from industrially available compounds that may be used, e.g., for the replacement of lilial.