By Brian M. Lawrence, Consultant
There is a taxonomic controversy related to the most acceptable taxonomic name for lemon verbena. This author prefers the following Aloysia citriodora Palau [syn. A. triphylla (L’Herit) Britton, Lippia citriodora (Lam.) O. Kuntze, L.triphylla (L’Herit) O. Kuntze]. A summary of the oil composition found in the previous reviews (Lawrence 1976, 1977, 1988, 1998, 2004, 2008). The compounds, which are listed in elution order from a non-polar capillary GC column, were as follows:
(E)-2-hexenal (<0.1–0.2%)
(Z)-3-hexenol (<0.1%)
α-thujene (<0.1–0.2%)
α-pinene (<0.1–1.5%)
1-octen-3-ol (0.4–1.0%)
sabinene (0.2–3.8%)
β-pinene <0.1–0.5%
6-methyl-5-hepten-2-one (0.2–7.4%)
myrcene (0.1–0.7%)
3-octanol (0.1–0.4%)
α-terpinene (<0.1–0.1%)
p-cymene (<0.1–0.1%)
limonene (4.2–22.7%)
1,8-cineole (0.1–12.9%)
(Z)-β-ocimene (0.1–0.3%)
δ-terpinene (<0.1–1.0%)
(E)-β-ocimene (0.1–6.7%)
cis-sabinene hydrate (0.1–1.0%)
linalool (0.1–1.3%)
trans-sabinene hydrate (0.6–0.8%)
perillene (<0.1–0.1%)
α-thujone (0.2–0.5%)
α-campholenal (<0.1–0.1%)
cis-p-mentha-2,8-dien-1-ol (0.2–0.4%)
cis-limonene oxide (<0.1–0.7%)
trans-limonene oxide (<0.1–1.0%)
citronellal (0.1–1.3%)
terpinen-4-ol (<0.1–2.3%)’
(Z)-3-hexenyl butyrate (<0.1–0.4%)
α-terpineol (0.2–2.5%)
β-cyclocitral (<0.1–1.4%)
trans-carveol (0.1–0.4%)
citronellol (<0.1–1.0%)
nerol (0.1–5.2%)
cis-carveol (<0.1–0.1%)
hexyl 2-methylbutyrate (<0.1–0.3%)
neral (6.0–29.6%)
carvone (0.2–0.8%)
piperitone (<0.1–2.4%)
geraniol (<0.1–6.0%)
methyl citronellate (<0.1–0.5%)
geranial (10.8–38.3%)
γ-elemene (0.1–0.3%)
eugenol (<0.1–0.2%)
citronellyl acetate (<0.1–0.6%)
neryl acetate (0.1–4.0%)
α-copaene (0.1–1.1%)
geranyl acetate (1.0–9.3%)
β-bourbonene (0.4–1.2%)
β-cubebene (0.3–0.6%)
methyl eugenol (<0.1–0.1%)
α-cedrene (0.2–0.4%)
β-caryophyllene (0.3–4.3%)
cis-thujopsene (<0.1–0.2%)
aromadendrene (<0.1–0.6%)
α-himachalene (<0.1–0.7%)
α-humulene (0.2–0.5%)
(E)-β-farnesene (<0.1–0.1%)
geranyl propionate (<0.1–0.4%)
germacrene D (0.8–1.5%)
ar-curcumene (0.4–5.7%)
zingiberene (<0.1–0.8%)
α-muurolene (0.1–2.9%)
β-bisabolene (<0.1–0.5%)
δ-cadinene (0.2–1.9%)
γ-cadinene (0.1–1.9%)
(E)-nerolidol (0.2–2.0%)
spathulenol (0.7–8.9%)
caryophyllene oxide (0.9–6.6%)
viridiflorol (<0.1–0.1%)
T-cadinol (0.1–1.1%)
An oil produced in 0.22% yield from lemon verbena that was collected in full flower from an experimental garden in Campinas (Brazil) was subjected to an antimicrobial screening by Sartoratto et al. (2004). Using GC-FID and GC/MS as their method of analysis, the authors characterized the following constituents in this oil:
1-octen-3-ol (0.4%)
p-cymene (1.1%)
limonene (11.0%)
(E)-β-ocimene (0.4%)
δ-terpinene (0.5%)
linalool (1.1%)
α-terpineol (0.5%)
neral (17.5%)
geranial (21.8%)
geranyl acetate (3.3%)
1,7-di-epi-α-cedrene† (0.3%)
β-caryophyllene (6.7%)
ar-curcumene (5.1%)
zingiberene (0.5%)
γ-cadinene (0.6%)
spathulenol (4.3%)
caryophyllene oxide (7.0%)
† identity requires corroboration
A commercial oil of lemon verbena was analyzed by De Martino et al. (2009) using GC-FID and GC/MS. The constituents characterized in this oil were:
α-pinene (0.2%)
sabinene (0.5%)
1-hepten-3-one (0.2%)
o-cymene (0.1%)
limonene (2.3%)
1,8-cineole (0.4%)
β-phellandrene (0.7%)
(E)-β-ocimene (0.3%)
δ-terpinene (0.1%)
linalool (0.1%)
isopinocamphone (0.2%)
borneol (0.1%)
terpinen-4-ol (0.2%)
α-terpineol (0.3%)
isobornyl formatea (45.4%)
(Z)-anethole† (0.2%)
geranial (44.5%)
α-copaene (0.2%)
isoledene† (0.1%)
β-elemene (0.2%)
β-caryophyllene (0.1%)
β-cedrene† (0.4%)
α-humulene (0.2%)
allo-aromadendrene (0.1%)
bicyclogermacrene (0.1%)
cis-muurola-4(14),5-diene (0.2%)
7-epi-α-selinene (0.2%)
a must be neral
† incorrect identification
In addition, β-pinene, α-terpinene, (Z)-β-ocimene, terpinolene, trans-pinocarveol, pinocamphone, pinocarvone, p-cymen-8-ol, bornyl acetate, isobornyl acetate, methyl eugenol, longifolene and δ-gurjunene were also listed as trace (<0.05%) constituents of this commercial oil. Also, the unnatural ratio of geranial to neral reveals that the oil used in this study was adulterated.
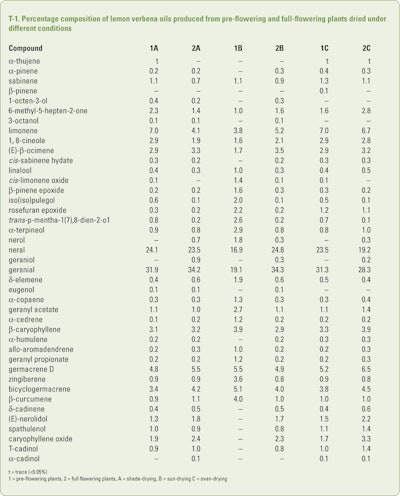
Lemon verbena plants that were grown in a greenhouse (25°/13°c day/night temperatures and 65% relative humidity) in Sepidan city (Shiraz, Iran) were harvested either pre-flowering or when the plants were in full flower by Agah and Najafian (2012a and 2012b). Once harvested the aerial part were dried using shade, full sun or in an oven at 60°c for 24hrs. The dried pre-flowering and full flowering plants were subjected to separate hydrodistillation for 3hrs. As expected the yields for the dried pre-flowering plants were 1.35% (shade), 1.2% (sun) and ca 1.1% (oven) while the yields for the dried full-flowering plants were 1.0–1.2% (shade), 1.1% (sun) and 0.5–0.8% (oven). Each oil was analyzed using GC/MS only, the results of which can be seen in T-1.
An oil of lemon verbena that was produced from plants grown in Colombia was screened for its antiviral activity against the yellow fever virus by Gomez et al. (2013). The main constituents of this oil, which were determined using GC/MS and GC-FID, were found to be:
limonene (10.7%)
1,8-cineole (5.0%)
nerol (2.0%)
neral (15.6%)
geraniol (2.7%)
geranial (18.9%)
geranyl acetate (1.3%)
β-caryophyllene (2.3%)
spathulenol (4.7%)
Hudaib et al. (2013) examined a lab-distilled oil of lemon verbena produced from plants grown in Jordan using GC-FID and GC/MS and retention indices. The constituents characterized in this oil were:
α-thujene (0.2%)
α-pinene (1.3%)
sabinene (3.5%)
β-pinene (0.2%)
1-octen-3-ol (0.1%)
6-methyl-5-hepten-2-one (0.6%)
myrcene (0.5%)
α-terpinene (0.1%)
p-cymene (0.1%)
limonene (17.7%)
1,8-cineole (11.7%)
(E)-β-ocimene (1.9%)
δ-terpinene (0.1%)
cis-sabinene hydrate (1.3%)
terpinolene (0.1%)
linalool (0.4%)
trans-sabinene hydrate (0.2%)
α-pinene oxide + α-thujone (0.4%)
trans-p-mentha-2,8-dien-1-ol (0.4%)
α-campholenal (0.1%)
cis-limonene oxide (0.3%)
trans-p-menth-2-en-1-ol (0.4%)
trans-verbenol (0.3%)
trans-chrysanthenal† (0.7%)
citronellal (0.2%)
cis-chrysanthenol† (0.5%)
rosefuran epoxide (0.3%)
terpinen-4-ol (0.4%)
(E)-isocitral (0.7%)
α-terpineol (0.3%)
trans-p-mentha-1(7),8-dien-2-ol (0.2%)
δ-terpineola (3.4%)
myrtenol (0.1%)
trans-carveol (0.3%)
cis-carveol (0.1%)
neral (9.8%)
carvone (0.2%)
piperitone (0.3%)
geranial (10.1%)
γ-elemene (0.1%)
α-copaene (0.4%)
geranyl acetate (0.3%)
β-bourbonene (0.5%)
β-cubebene (0.1%)
methyl eugenol (0.3%)
α-cedrene (0.5%)
β-caryophyllene (2.6%)
β-cedrene (0.5%)
β-copaene (0.2%)
α-humulene (0.4%)
allo-aromadendrene (0.7%)
β-acoradiene (0.4%)
δ-muurolene (0.3%)
ar-curcumene (6.3%)
zingiberene (0.6%)
bicyclogermacrene (1.6%)
α-cuprenene (0.8%)
δ-cadinene (0.3%)
γ-cadinene (0.2%)
α-cadinene (0.2%)
(E)-nerolidol (0.5%)
spathulenol (4.6%)
caryophyllene oxide (3.1%)
helifolen-12-al A† (0.3%)
6-(p-tolyl)-2-methyl-2-heptenolb (0.5%)
cedrol (0.2%)
epi-cedrol (0.8%)
allo-aromadendrene epoxide (0.4%)
α-muurolol (1.1%)
allo-himachalol (0.3%)
intermedeol (0.3%)
khusinol (0.2%)
† identity requires corroboration
a does not occur in nature
b contaminant not a natural constituent
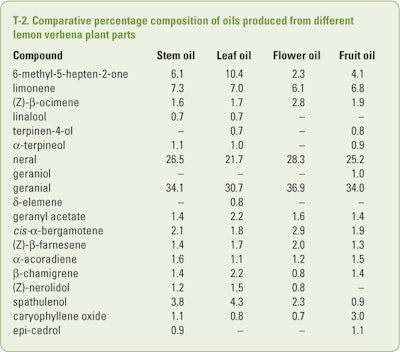
Shahhoseini et al. (2014) examined the composition of lab-distilled oils produced from stems, leaves, flowers and fruit of lemon verbena growing in an experimental garden at Tarbiat Modares University (Tehran, Iran) using GC-FID and GC/MS. The results of this study are summarized in T-2. In addition, trace amounts (<0.1%) of α-thujone, trans-carvyl acetate, piperitenone, α-cubebene, β-sesquiphellandrene and trans-calamenene were characterized in one of the oils.
Lemon verbena plants grown either in the Nile Delta or Upper Egypt that were harvested at their full flowering stage were divided into small pieces and separately hydrodistilled by Ibrahim et al. (2014). Oils produced separately from each site were analyzed using GC-FID and GC/MS. The oil compositions were found to range as follows:
tricyclene (0.1%)
α-pinene (1.8%)
β-pinene (1.3%)
decane (0.3%)
limonene (15.4–16.4%)
1,8-cineole (7.5–9.6%)
δ-terpinene (2.0%)
linalool (0.9–1.0%)
borneol (0.3%)
α-terpineol (0.2%)
nerol (0.3%)
citronellol (3.3–3.4%)
neral (10.3–11.3%)
geraniol (3.1%)
geranial (13.0–15.0%)
α-cubebene (0.3%)
α-longipinene† (0.1%)
α-cedrene (0.3%)
β-caryophyllene (3.7–3.9%)
α-humulene (0.2%)
(E)-β-farnesene (0.5–0.6%)
ar-curcumene (3.6%)
1-(1,5-dimethyl-4-hexenyl)-4-methylbenzene† (4.9–5.0%)
caryophyllene oxide (2.9%)
(E,E)-farnesal† (0.6%)
† incorrect identification
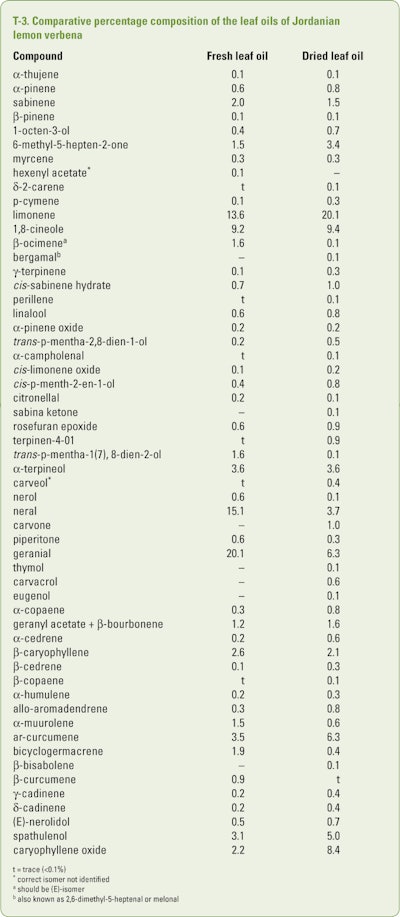
Two lab-distilled oils of fresh and dried leaves of lemon verbena that were produced by hydrodistillation for 3hr from plants grown in Jordan were analyzed using GC/MS and retention indices by Abuhamdah et al. (2015). The compositions of the two oils can be seen in T-3.
Lemon verbena that was cultivated in a green house in Iran was harvested when in full flower by Sardashti et al. (2015). An oil which was produced in 0.25% by hydrodistillation for 3hr was analyzed by using GC/MS only. The constituents characterized in the oil that was produced from dried plant material (listed in elution order from a polar column) were as follows:
α-pinene (0.3%)
sabinene (1.1%)
6-methyl-5-hepten-2-one (3.7%)
myrcene (0.2%)
limonene (6.4%)
1,8-cineole (4.4%)
(Z)-β-ocimene (0.2%)
(E)-β-ocimene (1.6%)
trans-sabinene hydrate (0.6%)
cumin alcohol (0.3%)
terpinolene (0.8%)
trans-p-mentha-2,8-dien-1-ol (0.1%)
(E)-salvene† (0.2%)
cis-p-mentha-2,8-dien-1-ol (0.1%)
trans-limonene oxide (0.1%)
trans-chrysanthemal† (0.4%)
citronellal (0.4%)
trans-carveol (0.5%)
γ-terpineol (0.2%)
rosefuran epoxide (1.3%)
pulegone† (0.7%)
β-fenchyl alcohol (1.8%)
neral (19.3%)
piperitone (0.2%)
geranial (23.0%)
piperitenone (0.1%)
eugenol (0.2%)
geranyl acetate (2.2%)
β-bourbonene (0.3%)
α-cedrene (0.4%)
β-caryophyllene (4.7%)
α-humulene (0.4%)
aromadendrene (0.4%)
β-farnesene* (0.1%)
neryl acetate (0.6%)
β-cubebene (4.1%)
curcumene* (2.5%)
bicyclogermacrene (2.7%)
di-epi-α-cedrene (1.9%)
γ-cadinene (0.3%)
caryophyllene oxide (8.7%)
γ-gurjunene† (0.4%)
isospathulenol (0.4%)
epi-zonarene (1.2%)
* correct isomer not identified
† incorrect identification
In addition, trace amounts (<0.05%) of a methylcyclohexane isomer, 3-methyl-2-butenal, methyl 2-methylbutyrate, a 3-hexenol isomer, a 2-hexenol isomer, α-thujene, camphene, ethyl-3-3-methylcyclopentene, 2,6-dimethyl-5-heptenal, δ-terpinene, trans-sabinene hydrate, p-vinyl guaicol and trans-carvyl acetate were also found in this oil.
The authors also examined an oil produced from fresh plant material. In this oil, the neral and geranial contents were 14.7% and 16.6%, respectively. Additional constituents characterized in this oil that were not detected in the oil produced from dried plant material were:
rosefuran (0.3%)
p-menth-8-en-3-one (0.3%)
2-caren-4-ol (0.3%)
isopulegone (0.3%
trans-p-mentha-1(7), 8-dien-2-ol-(0.8%)
eucarvone (<0.1%)
α-muurolene (<0.1%)
geranyl propionate (0.4%)
zingiberenol* (0.1%)
T-cadinol (0.2%)
*correct isomer not identified