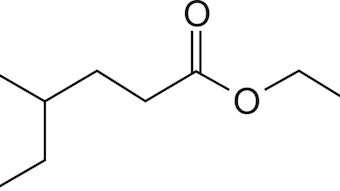
Some recent synthetic work will illustrate how these powerful reactions provide substrates that can be elaborated into novel polycyclic structures with interesting odor profi les. In the course of this research investigation, three new proprietary fragrance molecules were discovered and commercialized: Cassiffi x, a fresh herbal, long-lasting cassis note; Prismantol, a woody, spicy, ginger, cardamom note; and Prismylate, a woody, amber, vetivert odorant. These are presented in F-2.2,3
Background Research in Pursuit of Cassiffix
The Diels-Alder reaction was discovered in 1928 while Kurt Alder was working with Otto Diels. They showed that when butadiene, a diene, is allowed to react with acrolein, a dienophile, under thermal conditions, it produces a (4 + 2) cycloaddition product — 3-cyclohexene carboxaldehyde (F-3) — in high yields, thus offering a simple way to prepare cyclic structures. This reaction played a crucial role in the synthesis of reserpine, morphine and cantharedine, to name a few. Since its discovery, the Diels-Alder reaction has been used in the synthesis of numerous complex natural products. In fact, the Nobel Prize in chemistry was awarded to Kurt Alder and Otto Diels in 1950 because of the enormous utility of their discovery. For the complete article, click on "Purchase this article."