Near-infrared (NIR) spectroscopy, which is related to the first experiments performed by Herschel in 1800, has been widely used in agricultural and food sectors. The first methods in this specialized field of application were developed by Norris et al. in 1951. However, a real breakthrough of NIR spectroscopy as a routine technique was first obtained in the early 1980s, when efficient chemometric algorithms were successfully introduced.
Generally, the statistical support improved the interpretation of the NIR data dramatically and contributed strongly to this spectroscopy tool becoming one of today’s fastest growing analytical technologies. While numerous NIR spectroscopy methods have been established in the field of agriculture for more than four decades, IR (infrared) spectroscopy has been primarily used as a structure-elucidation technique; only limited application can be found in literature for characterization of liquid samples, such as fruit juices or other plant extracts. Until recently, Raman spectroscopy was restricted mostly to academic research. However, in the last 10 years, the development of Fourier transform methods involving the replacement of the dispersive element with an interferometer gained broader industrial application. Most attempts to get specific Raman signals from plant tissues failed because fluorescence, with a quantum yield several orders of magnitude larger than that of the Raman effect, concealed the whole Raman spectrum. Using excitation by an Nd:YAG laser at 1064 nm disturbing fluorescence of the enzymes and coenzymes, resulting usually by application of all other Raman techniques with excitation wavelengths in the visible range and even at 785 as well as 830 nm, is avoided.
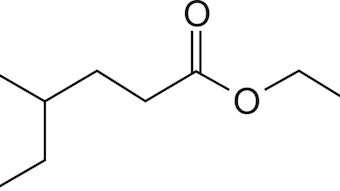
Generally, IR, NIR and Raman spectra of essential oils can be observed as a consequence of molecular vibrations of the individual components (e.g. mono- and sesquiterpenes, alcohols, aldehydes, aromatic substances) present in the volatile fraction. Due to the different excitation conditions of the mentioned spectroscopic techniques, the relationship between absorption intensities and the addressed functionalities of the analytes vary signifi cantly.