The first article in this series addressed the question of how to find fragrance chemicals that can be successfully incorporated into a specific polymer (polyvinylchloride). This paper will consider the opposite situation—given a specific fragrance, how can a polymer be selected to contain it?
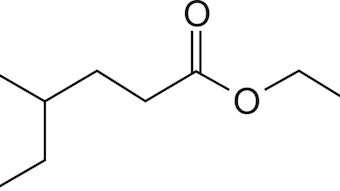
The fragrance that will be considered is vanilla because of the warm feeling it can give to many products. Also, the vanilla fragrance is produced by chemicals with strong hydrogen bonding ability and a Lewis acid character, which will provide contrast against the results obtained from perfume chemicals with moderate hydrogen bonding ability and Lewis base character discussed the last time. Also, vanilla has a low vapor pressure which demands heroic measures to get enough vanilla into a composition to get any appreciable “lift-off.”
As a further departure from the prior paper, the type of polymer host considered is one that can be fabricated into a label adhesive. Labels generally have large surface areas available for radiating fragrance, and they are the only opportunity to influence a customer that doesn’t open bottles in the store.